Question
Question: The reaction sequence shown below is used to synthesize benzamide. What is the structure of the fina...
The reaction sequence shown below is used to synthesize benzamide. What is the structure of the final product?
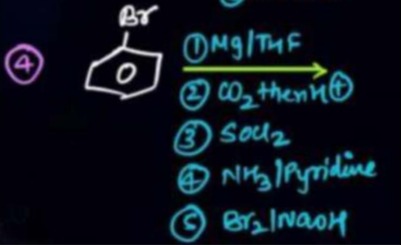
A
Benzene
B
Benzoic acid
C
Benzoyl chloride
D
Benzamide
Answer
Benzamide
Explanation
Solution
The reaction sequence involves the following steps:
- Grignard Reagent Formation: Bromobenzene reacts with magnesium in anhydrous THF to form phenylmagnesium bromide (C₆H₅MgBr).
- Carboxylation: Phenylmagnesium bromide reacts with carbon dioxide (CO₂) followed by acidic workup (H⁺) to yield benzoic acid (C₆H₅COOH).
- Acid Chloride Formation: Benzoic acid is treated with thionyl chloride (SOCl₂) to convert it into benzoyl chloride (C₆H₅COCl).
- Amide Formation: Benzoyl chloride reacts with ammonia (NH₃) in the presence of pyridine (which acts as a base) to form benzamide (C₆H₅CONH₂). The circled '4' next to the starting material suggests that the question is asking for the product formed after the 4th step of the reaction sequence, which is benzamide.
