Question
Question: [Figure] ...
[Figure]
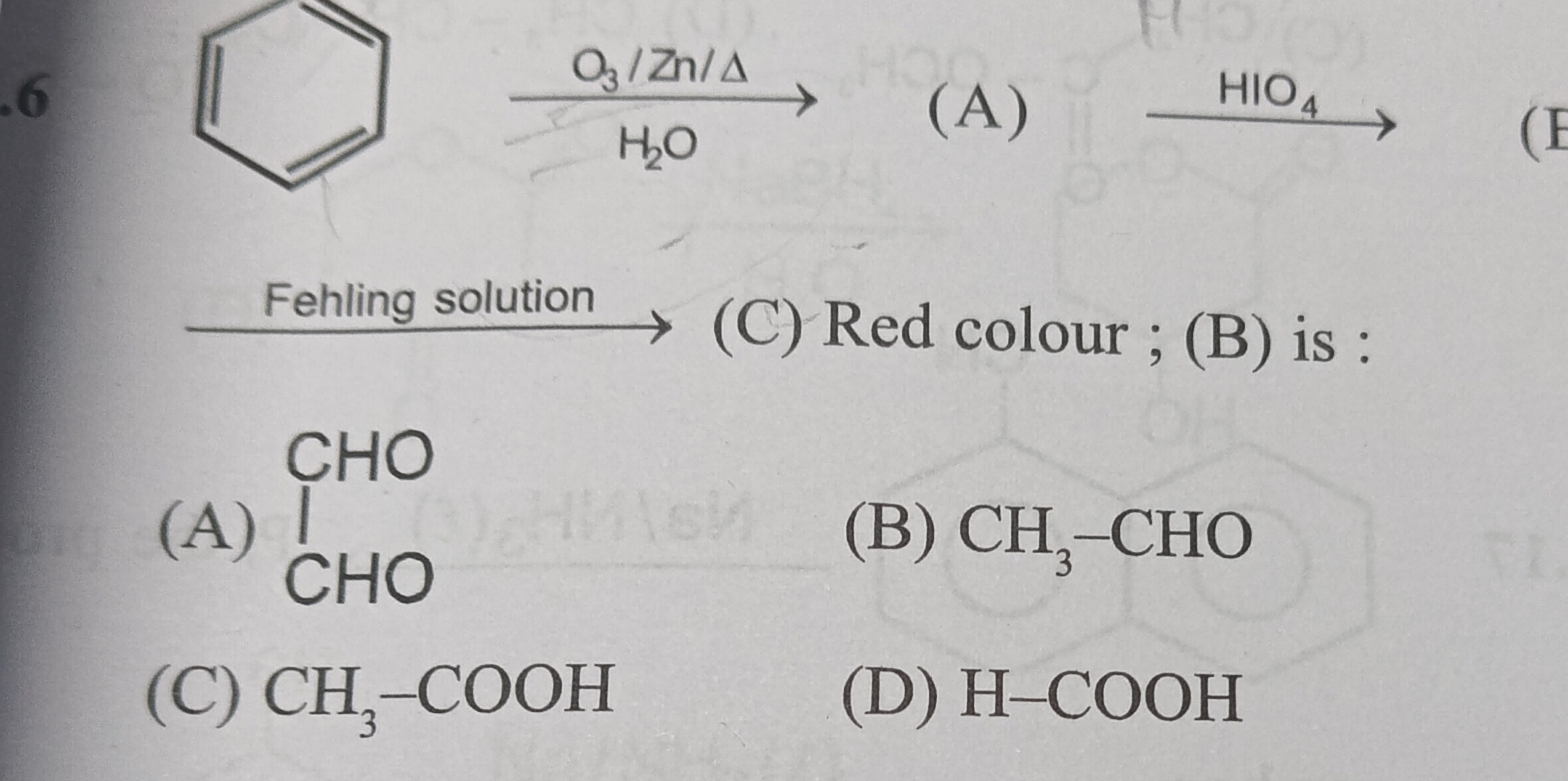
OHC-CHO
CH3-CHO
CH3-COOH
H-COOH
H-COOH
Solution
Step-by-step derivation:
-
Ozonolysis of Benzene:
The starting material is benzene. Reductive ozonolysis of benzene (O3/Zn/Δ,H2O) cleaves all three double bonds. This reaction converts each carbon-carbon double bond into two carbonyl groups. For benzene, this leads to the formation of three molecules of glyoxal.Therefore, product (A) is Glyoxal (OHC-CHO).
-
Reaction of (A) with Fehling's solution:
Product (A) is Glyoxal (OHC-CHO). Glyoxal contains two aldehyde groups. Aldehydes are oxidized by Fehling's solution (a mild oxidizing agent containing Cu(II) ions) to carboxylic acids, while the Cu(II) ions are reduced to Cu(I) oxide (Cu2O), which is a red precipitate.
The observation "Red colour" (C) confirms that (A) is an aldehyde and that the reaction with Fehling's solution is positive, forming Cu2O. -
Reaction of (A) with Periodic Acid (HIO4):
Product (A) is Glyoxal (OHC-CHO). Periodic acid (HIO4) is a reagent used for the oxidative cleavage of 1,2-diols, 1,2-diketones, 1,2-hydroxyaldehydes, and 1,2-dialdehydes. Glyoxal is a 1,2-dialdehyde.
The cleavage of a 1,2-dialdehyde by periodic acid results in the formation of carboxylic acids.
In the case of glyoxal (OHC-CHO), both aldehyde groups are on adjacent carbons. The cleavage occurs between these two carbons, and each carbon is oxidized to a carboxylic acid group.
OHC−CHOHIO4HCOOH+HCOOH
Therefore, product (B) is Formic acid (HCOOH).
Conclusion:
Based on the reaction sequence, (B) is Formic acid (HCOOH).
Benzene undergoes reductive ozonolysis to form glyoxal (OHC-CHO), which is product (A). Glyoxal, being an aldehyde, gives a positive Fehling's test (red precipitate (C)). Glyoxal (a 1,2-dialdehyde) is cleaved by periodic acid (HIO4) to produce two molecules of formic acid (HCOOH), which is product (B).
