Question
Question: The given reaction involves salicylic acid (2-hydroxybenzoic acid) reacting with phenol (PhOH) in th...
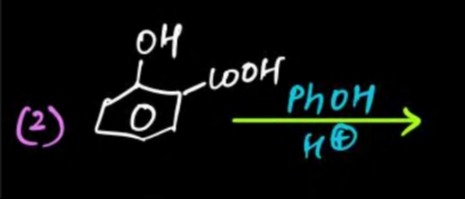
The given reaction involves salicylic acid (2-hydroxybenzoic acid) reacting with phenol (PhOH) in the presence of an acid catalyst (H⁺).
The product of the reaction is Phenyl Salicylate (Salol).
Solution
Salicylic acid contains two functional groups:
- A carboxylic acid group (-COOH)
- A phenolic hydroxyl group (-OH)
Phenol contains a phenolic hydroxyl group (-OH).
In the presence of an acid catalyst, a carboxylic acid reacts with an alcohol or a phenol to form an ester. This reaction is known as Fischer esterification.
The carboxylic acid group of salicylic acid will react with the hydroxyl group of phenol. The phenolic hydroxyl group of salicylic acid is less reactive and will remain unreacted under these conditions.
The reaction proceeds as follows: The -OH group from the carboxylic acid and a -H atom from the phenol's hydroxyl group are removed as a molecule of water (H₂O), forming an ester linkage.
The product formed is phenyl salicylate, commonly known as Salol.
Reaction:
Structures:
-
Reactant: Salicylic Acid (2-hydroxybenzoic acid) (SMILES: O=C(O)c1ccccc1O)
O=C(O) | c1-c2-c(O)-c(C=C)c1 -
Reagent: Phenol (PhOH) (SMILES: Oc1ccccc1)
OH | c1ccccc1 -
Product: Phenyl Salicylate (Salol) (SMILES: O=C(Oc1ccccc1)c2ccccc2O)
OH / c2-c1-c(C(=O)Oc3ccccc3)-c(C=C)c2
Explanation of the Solution:
Salicylic acid's carboxylic acid group undergoes acid-catalyzed esterification with phenol. The -COOH group loses -OH, and phenol loses -H (or vice versa, net loss of H₂O), forming an ester bond between the carbonyl carbon of salicylic acid and the oxygen of phenol. The phenolic -OH group of salicylic acid remains intact. The product is phenyl salicylate.
\begin{center} \includegraphics[width=0.4\textwidth]{salicylic_acid.png} + \includegraphics[width=0.2\textwidth]{phenol.png} \quad $\xrightarrow{\text{H}^{+}}$ \quad \includegraphics[width=0.4\textwidth]{phenyl_salicylate.png} + \text{H}_2\text{O} \end{center}
(Note: The images in the latex block above are placeholders and would need to be actual chemical structure diagrams or SMILES strings rendered as images for proper display.)
The structure of Phenyl Salicylate:
O=C(Oc1ccccc1)c2ccccc2O
