Question
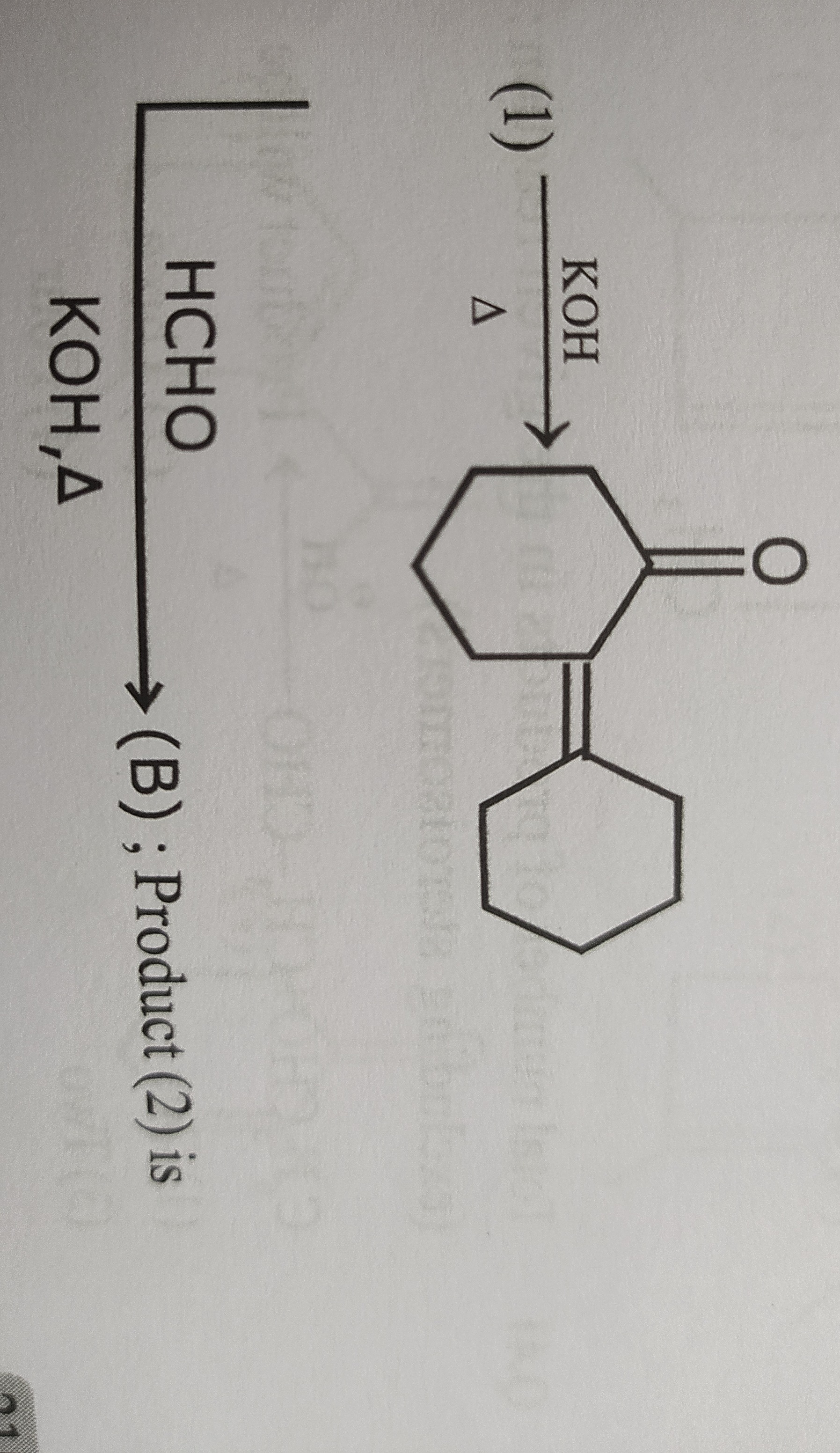
Question: The problem involves a two-step reaction sequence starting from cyclohexanone. **Step 1: Reaction (...
The problem involves a two-step reaction sequence starting from cyclohexanone.
Step 1: Reaction (1)
The starting material is cyclohexanone (O=C1CCCCC1).
Reagents: KOH, Δ (potassium hydroxide, heat).
This is a typical aldol condensation reaction. Under basic conditions and heating, two molecules of cyclohexanone undergo condensation.
- A base (KOH) deprotonates an alpha-hydrogen of one cyclohexanone molecule to form an enolate.
- The enolate acts as a nucleophile and attacks the carbonyl carbon of another cyclohexanone molecule to form a β-hydroxy ketone (aldol adduct).
- Under heating conditions (Δ), the β-hydroxy ketone undergoes dehydration to form an α,β-unsaturated ketone.
The product (A) formed from the aldol condensation of two cyclohexanone molecules followed by dehydration is 2-cyclohexylidenecyclohexanone.
The reaction is:
2 C6H10O (Cyclohexanone) --(KOH, Δ)--> O=C1CCCCC1=C2CCCCC2 (Product A) + H2O
Structure of Product (A):
O
//
C1
/ \
C6 C2
/ \ //
C5 C3
\ /
C4
||
C(of second ring)
SMILES: O=C1CCCCC1=C2CCCCC2
Step 2: Reaction (B) Reactant: Product (A) = 2-cyclohexylidenecyclohexanone Reagents: HCHO (formaldehyde), KOH, Δ. This is a crossed aldol condensation reaction with formaldehyde, followed by dehydration.
First, identify the acidic alpha-hydrogens in Product (A):
- The carbonyl carbon is C1.
- The alpha carbons are C2 and C6.
- Carbon C2 is part of the exocyclic double bond (
C2=C(second ring)), so it has no alpha-hydrogens. - Carbon C6 has two alpha-hydrogens. These are acidic and will be abstracted by the base (KOH).
Mechanism for reaction (B):
- Enolate formation: KOH deprotonates an alpha-hydrogen from C6 of Product (A) to form an enolate.
O // C1 / \ C6- C2 / \ // H C3 \ / C4 || C(of second ring)Product A + KOH <=> [Enolate at C6]- K+ + H2OThe enolate can be represented as:O- / \ C1=C6 / \ C5 C2 \ // C4 C3 || C(of second ring) - Nucleophilic attack: The enolate (carbanion at C6) attacks the electrophilic carbonyl carbon of formaldehyde (HCHO).
This forms a β-hydroxy ketone (aldol adduct).
The adduct is 6-(hydroxymethyl)-2-cyclohexylidenecyclohexanone.
O // C1 / \ C6-CH2OH C2 / \ // H C3 \ / C4 || C(of second ring) - Dehydration: Under heating conditions (Δ), the β-hydroxy ketone undergoes dehydration. The -OH group from the
CH2OH(beta carbon) and a hydrogen from the alpha carbon (C6) are removed, forming a new double bond. The final product (B) is 6-methylene-2-cyclohexylidenecyclohexanone.
Structure of Product (B):
O
//
C1
/ \
C6=CH2 C2
/ \ //
C5 C3
\ /
C4
||
C(of second ring)
SMILES: O=C1C(=C)CCCC1=C2CCCCC2
The final product (B) is 6-methylene-2-cyclohexylidenecyclohexanone.
The image provided only asks for "Product (2)", which corresponds to Product (B) in our analysis. The structure of Product (B) is shown above.
O=C1C(=C)CCCC1=C2CCCCC2
Solution
- Step 1 (Reaction 1): Cyclohexanone undergoes base-catalyzed aldol condensation followed by dehydration to form 2-cyclohexylidenecyclohexanone (Product A).
- Step 2 (Reaction B): Product A has acidic alpha-hydrogens only at C6. These hydrogens are abstracted by KOH to form an enolate. This enolate then attacks formaldehyde (HCHO) in a crossed aldol reaction. The resulting β-hydroxy ketone undergoes dehydration under heating (Δ) to form an α,β-unsaturated ketone, 6-methylene-2-cyclohexylidenecyclohexanone (Product B).
