Question
Question: ...
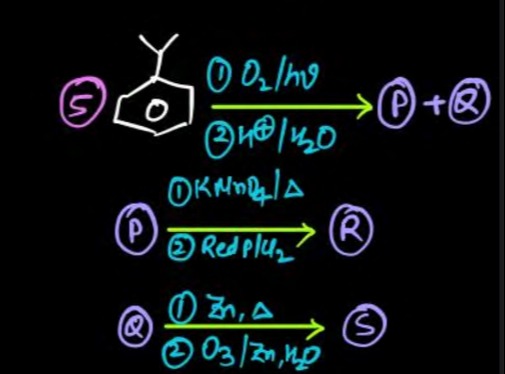
P = Phenol, Q = Acetone, R = Benzene, S = Isopropylbenzene
Solution
-
S to P + Q: Isopropylbenzene (S) undergoes cumene hydroperoxide process (oxidation with O2/hν followed by acid hydrolysis with H⊕/H2O) to yield phenol (P) and acetone (Q).
-
P to R: Phenol (P) is subjected to strong reduction with Red P/I2. This reagent deoxygenates phenol to benzene (R). The KMnO4/Δ step is a strong oxidation, but the subsequent strong reduction would convert any oxidized product back to benzene.
-
Q to S: Acetone (Q) is shown to convert to isopropylbenzene (S) using Zn, Δ followed by O3/Zn, H2O. This conversion is not chemically feasible with the given reagents. Ozonolysis is used for cleaving C=C bonds, which are absent in acetone. Therefore, this reaction sequence is inconsistent with standard organic chemistry. However, based on the initial structure provided, S is isopropylbenzene.
P (Phenol): Structure: C6H5-OH SMILES: Oc1ccccc1
Q (Acetone): Structure: CH3-CO-CH3 SMILES: CC(=O)C
R (Benzene): Structure: C6H6 SMILES: c1ccccc1
S (Isopropylbenzene): Structure: C6H5-CH(CH3)2 SMILES: CC(C)c1ccccc1
The reaction Q -> S is chemically unsound as written.
Answer:
P: Phenol (C6H5-OH) Q: Acetone (CH3-CO-CH3) R: Benzene (C6H6) S: Isopropylbenzene (C6H5-CH(CH3)2)
The reaction Q --(1) Zn, Δ ; (2) O3/Zn, H2O--> S is not a valid chemical transformation.
