Question
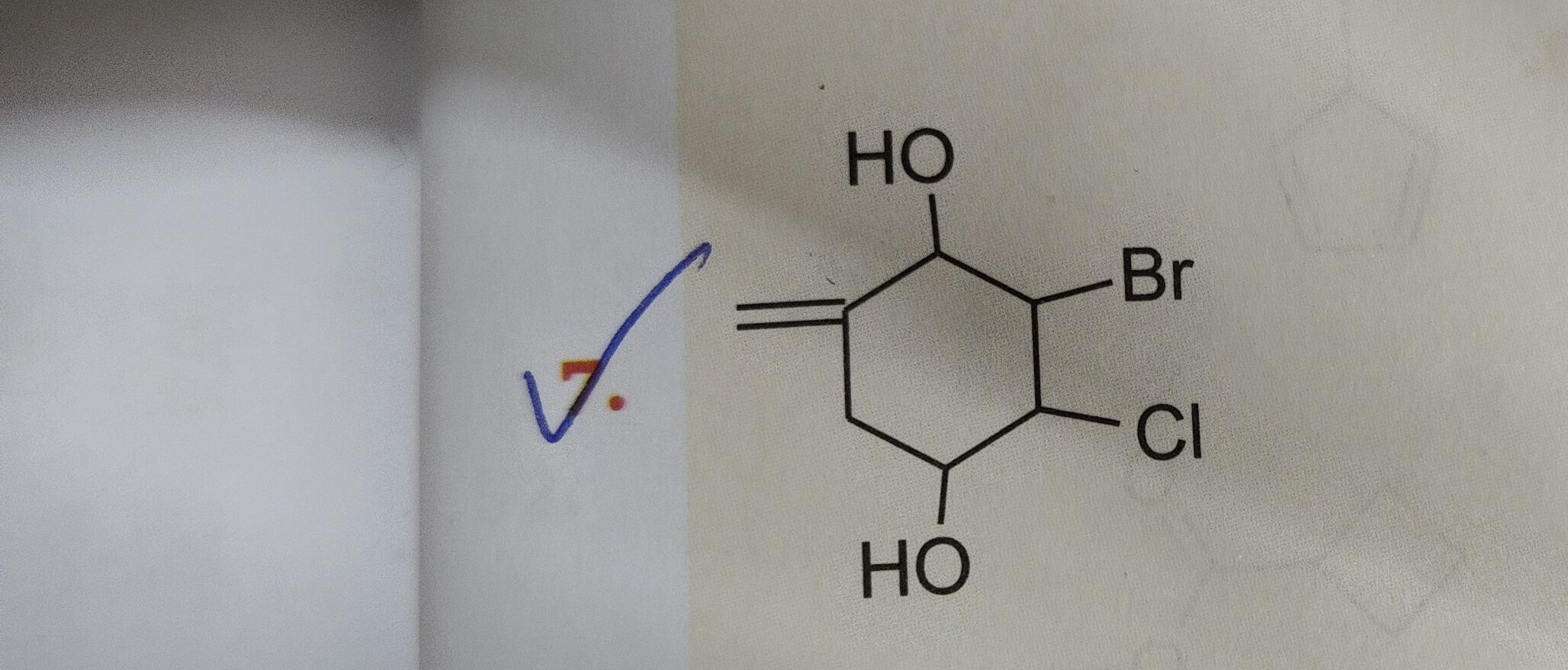
Question: The given structure is a substituted cyclohexene ring. To name it according to IUPAC rules, we follo...
The given structure is a substituted cyclohexene ring. To name it according to IUPAC rules, we follow these steps:
- Identify the parent chain/ring: The structure is a six-membered ring containing a double bond, so the parent is cyclohexene.
- Identify and prioritize functional groups: We have a double bond, two hydroxyl (-OH) groups, a bromine (-Br) atom, and a chlorine (-Cl) atom. Alcohols (-OH) have higher priority than halogens. The double bond is part of the parent ring. Since there are two -OH groups, the suffix will be "-diol".
- Number the ring:
- The carbons of the double bond must be numbered C1 and C2.
- Numbering should proceed in a direction that gives the lowest possible locants to the principal functional groups (the -OH groups in this case).
- If there's a tie, then assign the lowest locants to other substituents (halogens), and finally, alphabetical order is used.
Answer
4-bromo-5-chloro-1-cyclohexene-3,6-diol
Explanation
Solution
-
Identify the parent ring as cyclohexene due to the six-membered ring and one double bond.
-
Prioritize functional groups: Hydroxyl (-OH) groups are the principal functional groups, followed by the double bond. Halogens are treated as substituents.
-
Number the ring:
- Assign C1 and C2 to the carbons of the double bond.
- Number in the direction that gives the lowest locants to the hydroxyl groups. This leads to -OH groups at C3 and C6.
- The remaining substituents, -Br and -Cl, are then at C4 and C5, respectively.
-
Alphabetize and name: Combine the substituent names (bromo, chloro) in alphabetical order with their locants, followed by the parent ring name (cyclohexene) with the double bond locant, and finally the diol suffix with its locants.
