Question
Question: **Step-by-step Derivations:** **Step 1: Formation of Product A** The starting material is morpholi...
Step-by-step Derivations:
Step 1: Formation of Product A The starting material is morpholine, a secondary amine. It reacts with benzoyl chloride (Ph-CO-Cl) in the presence of dilute NaOH. This is a nucleophilic acyl substitution reaction, specifically an acylation of the amine. The nitrogen atom of morpholine acts as a nucleophile and attacks the carbonyl carbon of benzoyl chloride. The chloride ion is eliminated as a leaving group. The dilute NaOH is a base that neutralizes the HCl formed during the reaction, preventing the protonation of the amine and ensuring the reaction proceeds to completion.
The reaction forms an N,N-disubstituted amide.
Initial Reactant (Morpholine): O(CH2CH2)2NH
Reaction:
O(CH2CH2)2NH + Ph-CO-Cl --(dil. NaOH)--> O(CH2CH2)2N-CO-Ph + HCl
(The HCl is neutralized by NaOH)
Product A is N-benzoyl morpholine.
Smiles for A: O1CCN(C(=O)c2ccccc2)CC1
Mermaid diagram for A:
Step 2: Formation of Product B Product A (N-benzoyl morpholine) is an N,N-disubstituted amide. It reacts with methylmagnesium bromide (CH3MgBr), a Grignard reagent, followed by acidic workup (H3O+). N,N-disubstituted amides react with Grignard reagents to form ketones. The Grignard reagent (CH3MgBr) acts as a strong nucleophile, with the methyl group (CH3-) attacking the carbonyl carbon of the amide. This forms a tetrahedral intermediate. Upon acidic workup, the morpholine moiety is eliminated as a leaving group, and the carbonyl group reforms, leading to the formation of a ketone.
Reaction:
O(CH2CH2)2N-CO-Ph + CH3MgBr --(1. CH3MgBr / 2. H3O+)--> Ph-CO-CH3 + O(CH2CH2)2NH (morpholine)
The methyl group from CH3MgBr replaces the morpholine group on the carbonyl carbon.
Product B is acetophenone.
Smiles for B: CC(=O)c1ccccc1
Mermaid diagram for B:
Explanation of the solution:
- Morpholine, a secondary amine, undergoes nucleophilic acyl substitution with benzoyl chloride in the presence of a base (dilute NaOH) to form N-benzoyl morpholine (Product A), which is an N,N-disubstituted amide.
- N-benzoyl morpholine (Product A) then reacts with methylmagnesium bromide (a Grignard reagent). Grignard reagents react with N,N-disubstituted amides to form ketones. The methyl group from the Grignard reagent adds to the carbonyl carbon, and the morpholine group is eliminated upon acidic workup, resulting in the formation of acetophenone (Product B).
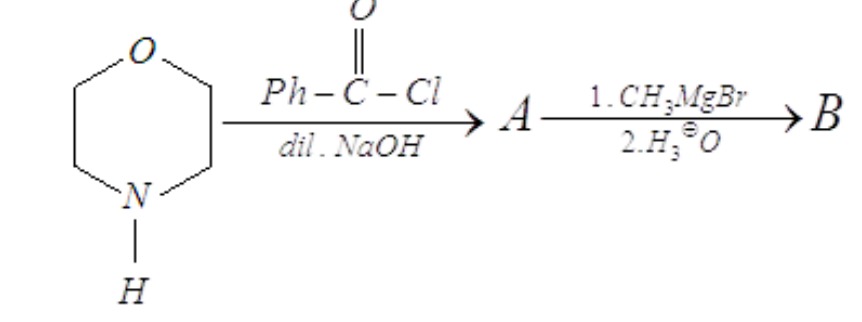
Acetophenone
Solution
The reaction sequence involves the formation of N-benzoyl morpholine (Product A) from morpholine and benzoyl chloride, followed by the reaction of Product A with methylmagnesium bromide and acidic workup to yield acetophenone (Product B).
