Question
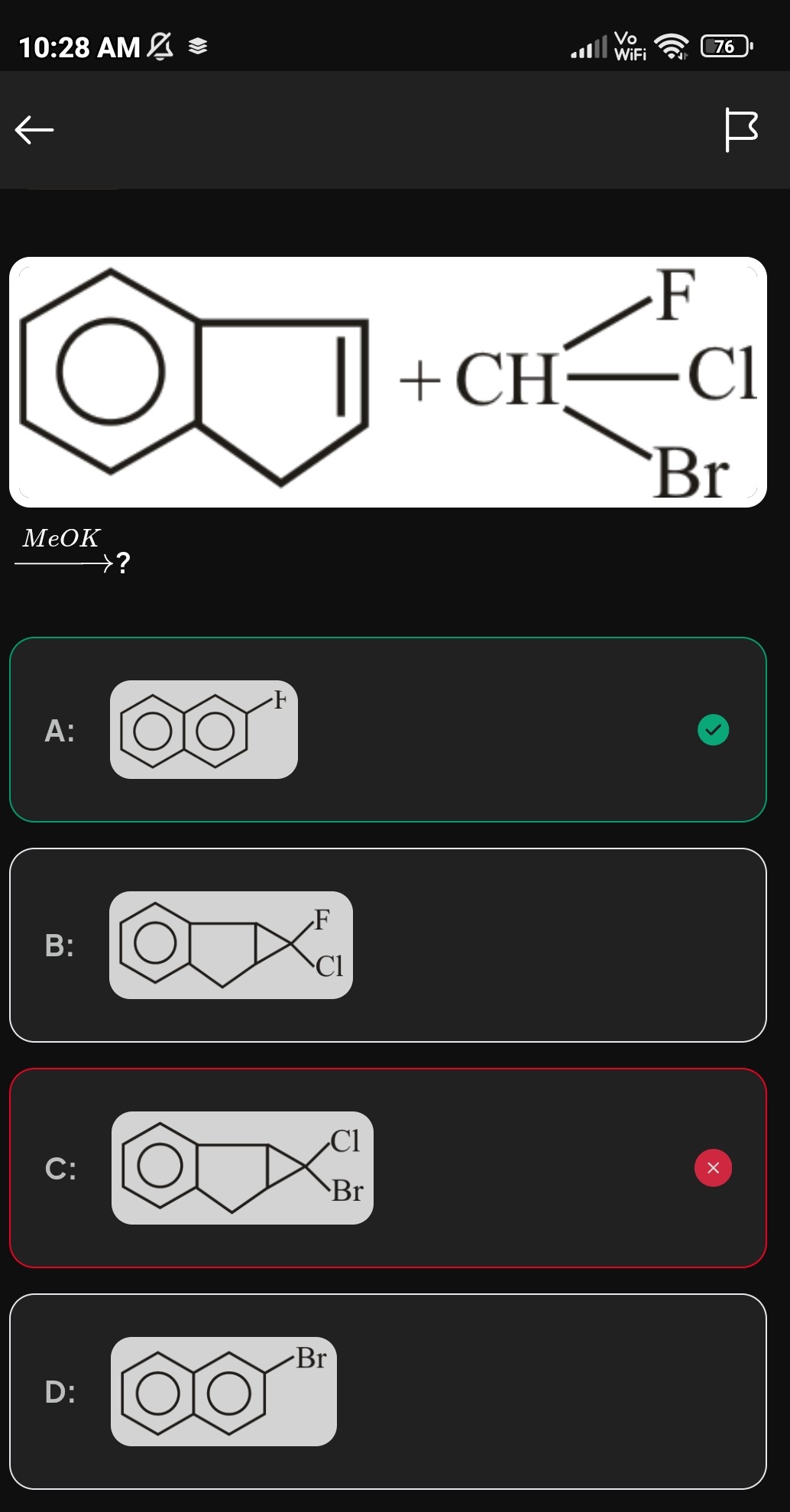
Question: The reaction involves indene, fluorochlorobromomethane (CH(F)(Cl)(Br)), and potassium methoxide (MeO...
The reaction involves indene, fluorochlorobromomethane (CH(F)(Cl)(Br)), and potassium methoxide (MeOK). What is the major product of this reaction?
2-Fluoronaphthalene.
A cyclopropane fused indene derivative with F and Cl on the cyclopropane carbon.
A cyclopropane fused indene derivative with Cl and Br on the cyclopropane carbon.
2-Bromonaphthalene.
A cyclopropane fused indene derivative with F and Cl on the cyclopropane carbon.
Solution
The reaction proceeds via carbene formation and subsequent cyclopropanation.
-
Carbene Generation: Potassium methoxide (MeOK) deprotonates CH(F)(Cl)(Br) to form a carbanion. This carbanion then undergoes alpha-elimination, losing the best leaving group (Br−) to form fluorochlorocarbene (:C(F)(Cl)). The order of leaving group ability is I > Br > Cl > F. CH(F)(Cl)(Br)+MeO−→−C(F)(Cl)(Br)+MeOH −C(F)(Cl)(Br)→:C(F)(Cl)+Br−
-
Cyclopropanation: The generated fluorochlorocarbene adds across the double bond of indene in a [1+2] cycloaddition reaction. This forms a new three-membered cyclopropane ring fused to the five-membered ring of indene, with the cyclopropane carbon bearing the fluorine and chlorine atoms.
Therefore, the major product is a cyclopropane fused indene derivative with F and Cl on the cyclopropane carbon.
