Question
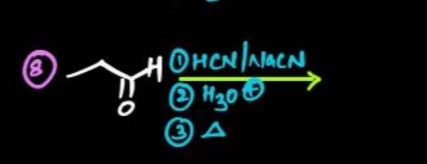
Question: Describe the reaction sequence starting with propanal (CH₃CH₂CHO) reacting with HCN in the presence ...
Describe the reaction sequence starting with propanal (CH₃CH₂CHO) reacting with HCN in the presence of NaCN, followed by hydrolysis under acidic conditions (H₃O⁺), and finally heating the product.
But-2-enoic acid
Solution
The reaction sequence involves three main steps:
Step 1: Nucleophilic addition of HCN to Propanal
Propanal (CH₃CH₂CHO) reacts with HCN in the presence of a base (like NaCN, which provides CN⁻ ions) to form cyanohydrins via a nucleophilic addition reaction. The cyanide ion (CN⁻) attacks the electrophilic carbonyl carbon, and the oxygen atom gets protonated.
\begin{center} CH_3CH_2CHO \quad \xrightarrow{HCN/NaCN} \quad CH_3CH_2CH(OH)CN \end{center}
The product formed is 2-hydroxybutanenitrile (also known as propanal cyanohydrin).
Step 2: Hydrolysis of the Nitrile group
The 2-hydroxybutanenitrile formed in step 1 contains a nitrile (-CN) group. Nitriles can be hydrolyzed under acidic conditions (H₃O⁺) to form carboxylic acids.
\begin{center} CH_3CH_2CH(OH)CN \quad \xrightarrow{H_3O^+} \quad CH_3CH_2CH(OH)COOH \end{center}
The product formed is 2-hydroxybutanoic acid.
Step 3: Dehydration of the alpha-hydroxy acid
The 2-hydroxybutanoic acid is an alpha-hydroxy acid (the hydroxyl group is on the carbon atom adjacent to the carboxyl group). When alpha-hydroxy acids are heated (Δ), they can undergo dehydration to form alpha,beta-unsaturated carboxylic acids. The hydroxyl group from the alpha-carbon and a hydrogen atom from the beta-carbon are eliminated as a molecule of water, creating a double bond between the alpha and beta carbons.
\begin{center} CH_3CH_2CH(OH)COOH \quad \xrightarrow{\Delta} \quad CH_3CH=CHCOOH + H_2O \end{center}
The final product is But-2-enoic acid (also commonly known as Crotonic acid). This product is stable due to the conjugation of the carbon-carbon double bond with the carboxyl group.
The final product is But-2-enoic acid.
Explanation:
-
Propanal undergoes nucleophilic addition with HCN/NaCN to form 2-hydroxybutanenitrile.
-
The nitrile group of 2-hydroxybutanenitrile is hydrolyzed under acidic conditions to a carboxylic acid group, yielding 2-hydroxybutanoic acid.
-
Upon heating, 2-hydroxybutanoic acid (an alpha-hydroxy acid) undergoes dehydration to form an alpha,beta-unsaturated carboxylic acid, which is But-2-enoic acid.
