Question
Question: ...
3,4-dihydrocyclobutane[1,2-c]furan-1,5-dione
Solution
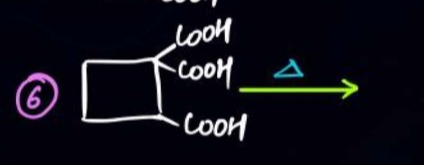
The reactant is a cyclobutane derivative with a -C(COOH)3 group at one carbon and a -COOH group at an adjacent carbon. Upon heating (Δ):
-
The -C(COOH)3 group undergoes two successive decarboxylations, losing 2 molecules of CO2, to form a -CH2COOH group. Reaction: R-C(COOH)3 Δ R-CH2COOH + 2CO2 The intermediate product is cyclobutane-1-(carboxymethyl)-2-carboxylic acid.
-
The intermediate product has a -CH2COOH group and a -COOH group on adjacent carbons of the cyclobutane ring. Upon further heating, these two carboxylic acid groups undergo intramolecular dehydration to form a cyclic anhydride. This forms a 6-membered anhydride ring fused with the cyclobutane ring.
The final product is 3,4-dihydrocyclobutane[1,2-c]furan-1,5-dione.
Structure of Reactant:
COOH
|
C_ext-COOH
|
C1
/ \
C4 C2-COOH
\ /
C3
(Where C1, C2, C3, C4 form the cyclobutane ring, and C_ext is an external carbon attached to C1)
Structure of Intermediate after Decarboxylation:
CH2COOH
|
C1
/ \
C4 C2-COOH
\ /
C3
Structure of Final Product (Anhydride):
This can also be represented as:
O=C1OC(=O)C2C(C1)C(C)C2
