Question
Question: ...
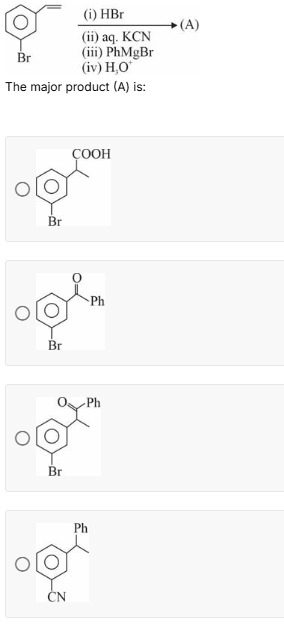
3
Solution
The reaction sequence involves four steps:
Step (i): Addition of HBr to the alkene
The starting material is 1-bromo-3-(prop-1-en-1-yl)benzene (or 3-bromostyrene). The alkene is a vinyl group attached to a benzene ring. Addition of HBr to an alkene follows Markovnikov's rule, meaning the hydrogen atom adds to the carbon with more hydrogen atoms, and the bromine atom adds to the more substituted carbon, leading to the formation of the more stable carbocation intermediate. For -CH=CH₂, H adds to the terminal CH₂ and Br adds to the internal CH, forming a secondary benzylic bromide which is resonance stabilized.
The product after step (i) is 1-bromo-3-(1-bromoethyl)benzene.
Step (ii): Nucleophilic substitution with aq. KCN
Aqueous KCN provides cyanide ions (CN⁻), which are strong nucleophiles. There are two bromine atoms in the molecule: one on the alkyl side chain (secondary benzylic bromide) and one on the benzene ring (aryl bromide). Aryl halides are generally unreactive towards nucleophilic substitution (SN1 or SN2) under normal conditions due to the sp² hybridization of the carbon bonded to bromine and resonance stabilization of the C-Br bond. The secondary benzylic bromide, however, is reactive towards SN2 (or SN1) reactions. The cyanide ion will displace the bromine atom on the side chain.
The product after step (ii) is 1-bromo-3-(1-cyanoethyl)benzene.
Step (iii) & (iv): Reaction with PhMgBr followed by H₃O⁺ hydrolysis
This is a standard reaction for the synthesis of ketones from nitriles using Grignard reagents. A nitrile (R-C≡N) reacts with a Grignard reagent (R'-MgX) to form an intermediate imine salt (R-C(R')=NMgX). Upon hydrolysis with H₃O⁺, this imine salt is converted into a ketone (R-C(=O)-R').
In our case, R is the (3-bromophenyl)-CH(CH₃)- group, and R' is the phenyl group (Ph) from PhMgBr. So, the -CN group is converted to a -C(=O)-Ph group, with the phenyl group (Ph) from PhMgBr attaching to the carbon that was originally part of the nitrile.
The final product (A) is 1-phenyl-2-(3-bromophenyl)propan-1-one.
Comparing this structure with the given options:
- Option 1 shows a carboxylic acid group (-COOH) instead of a ketone. (Incorrect)
- Option 2 shows a ketone directly attached to the benzene ring without the -CH(CH₃) part. (Incorrect)
- Option 3 perfectly matches the derived structure.
- Option 4 shows a nitrile group on the ring and a phenyl group on the side chain, which is incorrect. (Incorrect)
Therefore, option 3 is the correct major product.
Explanation of the solution:
- HBr addition: Markovnikov's addition of HBr to the styrene derivative yields 1-bromo-3-(1-bromoethyl)benzene.
- KCN substitution: The alkyl bromide undergoes SN2 reaction with KCN, replacing Br with CN. The aryl bromide remains unreactive. This gives 1-bromo-3-(1-cyanoethyl)benzene.
- Grignard reaction & Hydrolysis: The nitrile group reacts with PhMgBr, followed by hydrolysis (H₃O⁺), to form a ketone. The phenyl group from PhMgBr adds to the carbon of the nitrile, forming 1-phenyl-2-(3-bromophenyl)propan-1-one.
