Question
Question: The question illustrates an electrophilic addition reaction of an alkene with HBr, followed by a car...
The question illustrates an electrophilic addition reaction of an alkene with HBr, followed by a carbocation rearrangement and attack by a nucleophile. Let's break down the mechanism as depicted in the image.
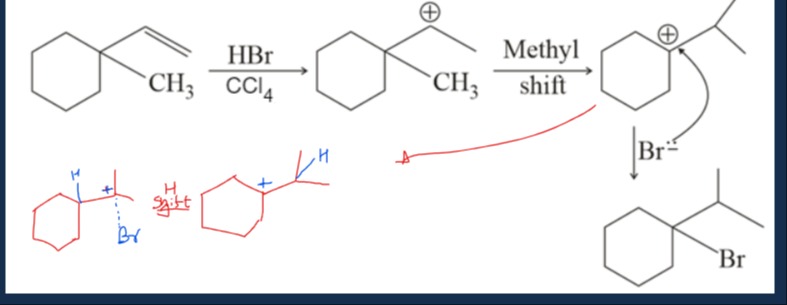
The mechanism illustrates an electrophilic addition reaction of an alkene, carbocation formation, a methyl shift rearrangement, and subsequent nucleophilic attack by Br- to form the final product. The final product is 1-bromo-1-isopropylcyclohexane.
Solution
The reaction starts with 1-(cyclohexyl)-1-methylethene reacting with HBr. This is an electrophilic addition to an alkene, forming a carbocation intermediate. The image depicts the initial carbocation as 1-cyclohexyl-1-methyl-1-ethyl carbocation. This carbocation undergoes a 1,2-methyl shift, where a methyl group from the exocyclic carbon migrates to an adjacent carbon on the cyclohexyl ring, and the positive charge shifts to that ring carbon. This rearrangement forms a more stable tertiary carbocation within the cyclohexyl ring, which is substituted with an isopropyl group. Finally, the bromide ion (Br-) attacks this rearranged carbocation, forming a C-Br bond and yielding the final product, 1-bromo-1-isopropylcyclohexane.
