Question
Question: A common reagent or reaction type that can react with acetoacetic ester, 1,3-butadiene, and cyclopen...
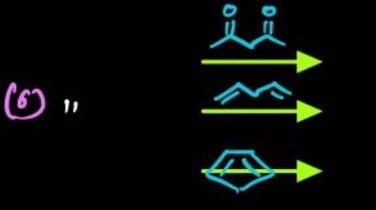
A common reagent or reaction type that can react with acetoacetic ester, 1,3-butadiene, and cyclopentadiene is:
Alpha,beta-unsaturated carbonyl compound
Solution
The three starting materials are: a β-keto ester (acetoacetic ester), 1,3-butadiene, and cyclopentadiene.
- The β-keto ester has an active methylene group, which can form a nucleophilic enolate. This enolate readily undergoes Michael addition with Michael acceptors.
- 1,3-Butadiene and cyclopentadiene are conjugated dienes, which are excellent substrates for the Diels-Alder reaction with appropriate dienophiles.
An alpha,beta-unsaturated carbonyl compound (e.g., methyl vinyl ketone) serves as both a Michael acceptor (due to the electrophilic β-carbon) and a dienophile (due to the electron-deficient double bond). Thus, it can react with all three given compounds through these distinct but compatible reaction types.
Reactions:
-
Reaction with Acetoacetic ester (Michael Addition): The enolate of acetoacetic ester (formed by deprotonation of the active methylene group) acts as a nucleophile and attacks the electrophilic β-carbon of the alpha,beta-unsaturated carbonyl compound (MVK). This is a classic Michael addition reaction, leading to the formation of a 1,5-dicarbonyl compound.
CH₃-CO-CH₂-COOEt + CH₂=CH-CO-CH₃ --(Base)--> CH₃-CO-CH(CH₂-CH₂-CO-CH₃)-COOEt -
Reaction with 1,3-Butadiene (Diels-Alder Reaction): 1,3-Butadiene acts as the diene, and the alpha,beta-unsaturated carbonyl compound (MVK) acts as the dienophile. They undergo a [4+2] cycloaddition (Diels-Alder reaction) to form a six-membered cyclic adduct.
CH₂=CH-CH=CH₂ + CH₂=CH-CO-CH₃ --> 3-acetylcyclohexeneThe product is a cyclohexene derivative with an acetyl group.
-
Reaction with Cyclopentadiene (Diels-Alder Reaction): Cyclopentadiene acts as the diene, and the alpha,beta-unsaturated carbonyl compound (MVK) acts as the dienophile. They undergo a [4+2] cycloaddition (Diels-Alder reaction) to form a bicyclic compound (a bicyclo[2.2.1]heptene derivative). Cyclopentadiene is particularly reactive in this reaction due to its locked cisoid conformation.
Cyclopentadiene + CH₂=CH-CO-CH₃ --> Bicyclo[2.2.1]heptene derivative (e.g., endo/exo-5-acetylbicyclo[2.2.1]hept-2-ene)
