Question
Question: Which of the following statement(s) is/are correct about given compounds?...
Which of the following statement(s) is/are correct about given compounds?
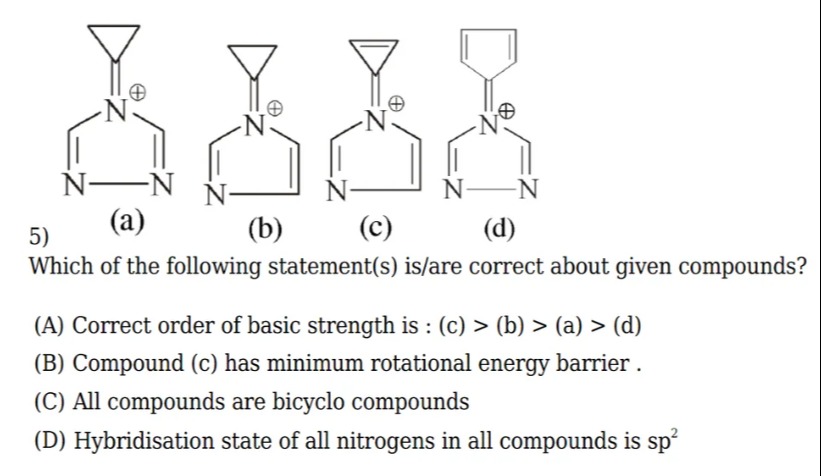
Correct order of basic strength is : (c) > (b) > (a) > (d)
Compound (c) has minimum rotational energy barrier.
All compounds are bicyclo compounds
Hybridisation state of all nitrogens in all compounds is sp²
A and D
Solution
The structures of the given compounds are:
(a) N-cyclopropyl-1,2,4-triazol-4-ium (b) N-cyclobutyl-1,2,4-triazol-4-ium (c) N-cyclopropenyl-1,2,4-triazol-4-ium (d) N-cyclopentadienyl-1,2,3-triazol-1-ium
Let's evaluate each statement:
(A) Correct order of basic strength is : (c) > (b) > (a) > (d).
(B) Compound (c) has minimum rotational energy barrier. This refers to rotation around the bond connecting the cyclopropenyl group to the nitrogen. The cyclopropenyl group has a double bond in the ring. Rotation around a single bond can be hindered by steric effects or electronic effects. In cyclopropenyl, the ring is rigid due to the double bond and the ring strain. Rotation around the N-C bond would involve rotating the entire cyclopropenyl ring relative to the triazole ring. The barrier to rotation depends on the change in energy during rotation.
(C) All compounds are bicyclo compounds. As discussed earlier, these compounds are not bicyclo compounds because the two rings share only one common atom.
(D) Hybridisation state of all nitrogens in all compounds is sp². In (a), (b), (c), and (d), the positively charged nitrogen is bonded to 3 atoms (external group and two ring atoms) and has no lone pair, so it is sp2 hybridized. Let's look at the other nitrogens in the 5-membered ring. In (a), (b), (c), the 5-membered ring is 1,2,4-triazol-4-ium. The ring has two double bonds. Let's assume the double bonds are between N-N and N=C. In 1,2,4-triazole, there are three nitrogen atoms. In the cation, the positive charge is on N4. The ring is aromatic with 6 pi electrons. All ring atoms are sp2 hybridized. So all nitrogens in the 1,2,4-triazole ring should be sp2 hybridized. In (d), the 5-membered ring is 1,2,3-triazol-1-ium. The ring has two double bonds. Let's assume the double bonds are between N-N and C=C. In 1,2,3-triazole, there are three nitrogen atoms. In the cation, the positive charge is on N1. The ring is aromatic with 6 pi electrons. All ring atoms are sp2 hybridized. So all nitrogens in the 1,2,3-triazole ring should be sp2 hybridized. Therefore, statement (D) appears to be correct.
