Question
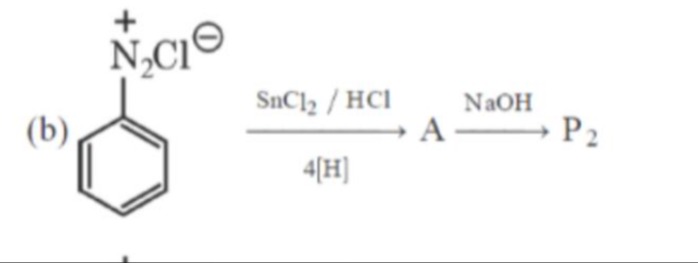
Question: + N₂Cl⁻ SnCl₂ / HCl NaOH (b) A P₂ 4[H] ...
N₂Cl⁻
SnCl₂ / HCl NaOH (b) A P₂ 4[H]
Answer
The final product P₂ is aniline (C₆H₅NH₂).
Explanation
Solution
We begin with an arenediazonium chloride (structure (b)) – i.e. a benzene ring with –N₂Cl attached. Under strongly reducing conditions with SnCl₂/HCl in the presence of “4[H]” the diazonium group is not simply lost as N₂ but is step‐wise reduced. In fact, the –N₂Cl group is converted by a 4‑electron (4H–atom) reduction into a phenylhydrazine intermediate
Ar–N2ClSnCl2/HCl, 4[H]Ar–NH–NH2(Product A)When this intermediate is subsequently treated with NaOH, a base–induced fragmentation occurs (the weak N–N bond is cleaved) so that ultimately an –NH₂ group replaces the hydrazino part. In other words:
Ar–NH–NH2NaOHAr–NH2(Product P2)Thus, the overall transformation is the conversion of the diazonium salt into an aniline derivative.
- Reduction: Arenediazonium chloride (b) is reduced with SnCl₂/HCl (using 4H–atoms) to yield phenylhydrazine (Product A).
- Base treatment: Phenylhydrazine reacts with NaOH causing N–N bond cleavage to form aniline (Product P₂).
