Question
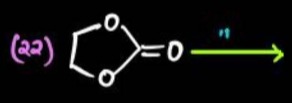
Question: The given reactant is ethylene carbonate (1,3-dioxolan-2-one). The symbol "n" above the arrow typica...
The given reactant is ethylene carbonate (1,3-dioxolan-2-one). The symbol "n" above the arrow typically indicates a polymerization reaction. Ethylene carbonate undergoes ring-opening polymerization, often with decarboxylation, to yield poly(ethylene oxide) and carbon dioxide.
Poly(ethylene oxide) and Carbon Dioxide
Solution
Ethylene carbonate is a cyclic carbonate. When subjected to polymerization conditions (often with a catalyst, not shown but implied by the 'n'), the ring opens. For ethylene carbonate, this polymerization commonly proceeds via decarboxylation, meaning the carbonyl group (C=O) is eliminated as carbon dioxide (\ceCO2), and the remaining \ce−CH2−CH2−O− units link together to form poly(ethylene oxide).
Reaction:
n \ce{O=C1OCCO1} \xrightarrow{\text{Polymerization}} \ce{(-CH2-CH2-O-)_n} + n \ce{CO2}
Reactant: Ethylene Carbonate
Product: Poly(ethylene oxide) and Carbon Dioxide
(Repeating unit of Poly(ethylene oxide))
