Question
Question: ...
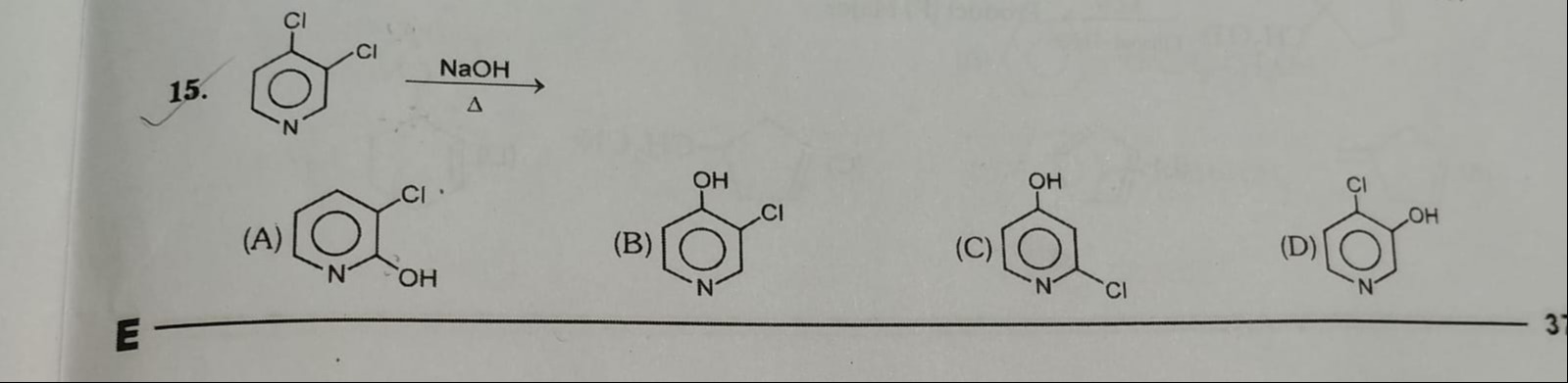
3-hydroxy-2-chloropyridine
2-hydroxy-3-chloropyridine
4-hydroxy-2-chloropyridine
3-hydroxy-4-chloropyridine
B
Solution
The given reaction is a nucleophilic aromatic substitution (S_NAr) of 2,3-dichloropyridine with sodium hydroxide (NaOH) under heating (Δ).
Understanding Pyridine Reactivity in S_NAr:
Pyridine is an electron-deficient aromatic heterocycle due to the electronegative nitrogen atom. This makes it susceptible to nucleophilic attack, especially at positions ortho (2 and 6) and para (4) to the nitrogen. The reason for this enhanced reactivity is the ability of the nitrogen atom to stabilize the negative charge developed in the Meisenheimer complex (the intermediate in S_NAr) through resonance. When the nucleophile attacks at position 2, 4, or 6, one of the resonance structures of the Meisenheimer complex places the negative charge directly on the nitrogen atom, which is highly stabilizing. Positions 3 and 5 are meta to the nitrogen, and nucleophilic attack at these positions does not allow the negative charge to be delocalized onto the nitrogen atom, making them less reactive towards S_NAr.
Analyzing 2,3-Dichloropyridine:
In 2,3-dichloropyridine, there are two chlorine atoms:
- Chlorine at position 2: This chlorine is at an ortho position relative to the nitrogen atom. This position is activated for nucleophilic substitution.
- Chlorine at position 3: This chlorine is at a meta position relative to the nitrogen atom. This position is not activated for nucleophilic substitution by the nitrogen atom.
Reaction Mechanism:
When NaOH (providing OH⁻ nucleophile) reacts with 2,3-dichloropyridine, the OH⁻ will preferentially attack the carbon bearing the more reactive chlorine atom. Based on the reactivity analysis, the chlorine at position 2 is significantly more reactive than the chlorine at position 3.
Therefore, the OH⁻ nucleophile will substitute the chlorine atom at position 2.
The product formed will be 2-hydroxy-3-chloropyridine. The hydroxyl group (OH⁻) from NaOH selectively replaces the chlorine at position 2, yielding 2-hydroxy-3-chloropyridine.
