Question
Question: ...
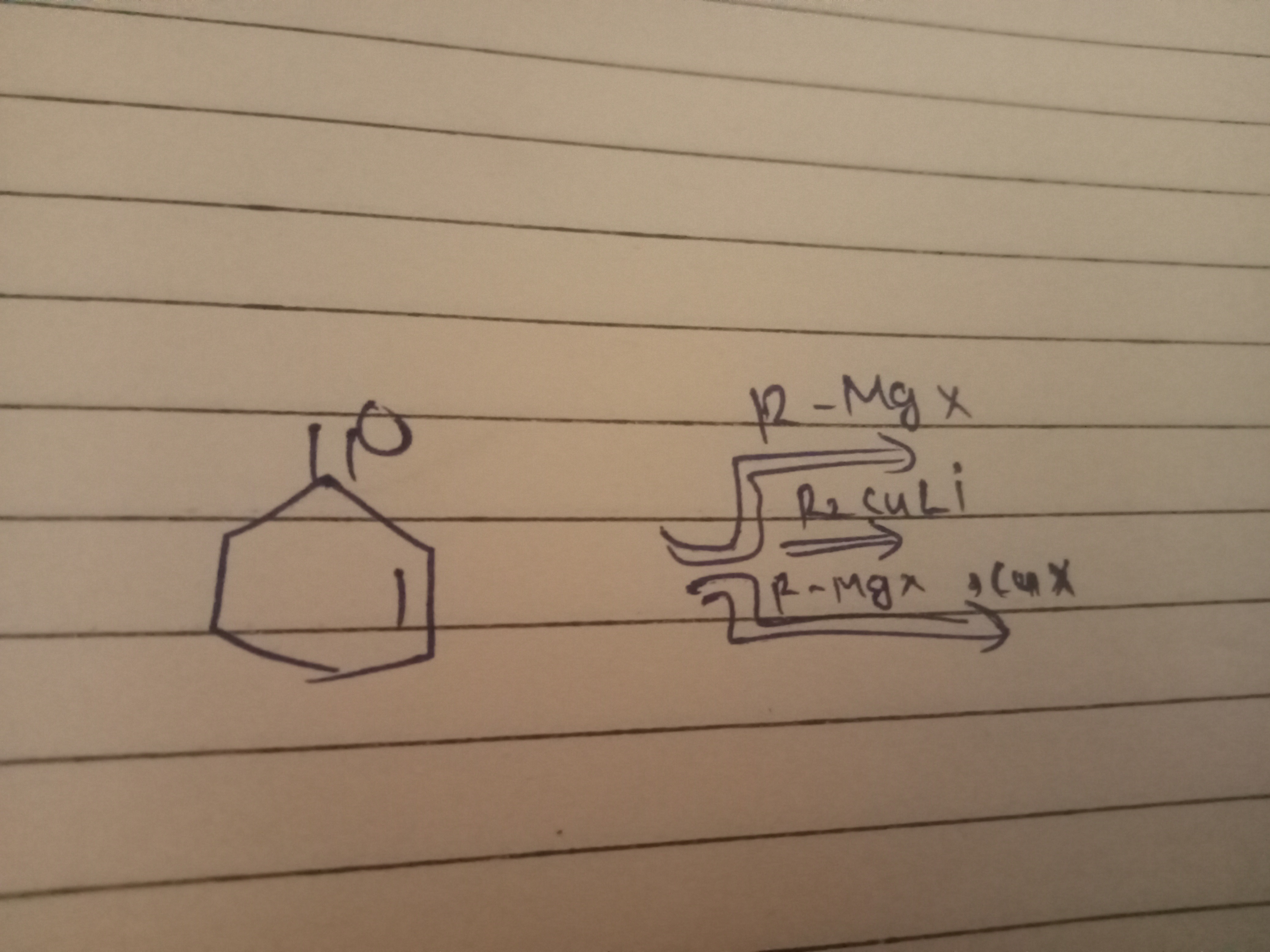
The product of the reaction is trans-2-alkylcyclohexanol.
Solution
Explanation of the Solution:
The starting material is cyclohexene oxide, a cyclic ether with significant ring strain, making it susceptible to nucleophilic ring-opening reactions.
-
Reaction with R-MgX (Grignard Reagent): Grignard reagents are strong carbon nucleophiles. They attack one of the carbons of the epoxide ring. Since cyclohexene oxide is symmetrical (both epoxide carbons are secondary and equivalent), the attack can occur at either carbon. The reaction proceeds via an SN2-like mechanism, where the nucleophile attacks from the backside relative to the C-O bond that breaks. This leads to the formation of an alkoxide intermediate, which upon acidic workup (protonation) yields an alcohol. The SN2 nature of the attack results in an inversion of configuration at the attacked carbon, leading to a trans relationship between the newly introduced alkyl group and the hydroxyl group.
-
Reaction with R₂CuLi (Gilman Reagent): Gilman reagents (lithium dialkylcuprates) are "softer" carbon nucleophiles than Grignard reagents. They also undergo SN2-like nucleophilic attack on epoxides, leading to ring opening. For symmetrical epoxides like cyclohexene oxide, the outcome is similar to that with Grignard reagents: a 2-substituted cyclohexanol is formed with trans stereochemistry.
-
Reaction with R-MgX + CuX (Copper-catalyzed Grignard Reaction): The addition of a copper(I) salt (CuX) to a Grignard reagent often modifies its reactivity, making it behave similarly to a Gilman reagent or facilitating specific reactions. In the case of epoxide opening, this combination also leads to an SN2-like nucleophilic attack, yielding the same trans-2-alkylcyclohexanol product as the other two reagents.
In all three cases, the product formed from the ring opening of cyclohexene oxide by an organometallic reagent (R-group) is trans-2-alkylcyclohexanol.
The general structure of the product is:
OH
|
C
/ \
C---C-R
/ \
C C
\ /
C---C
(where the -OH group and the -R group are in a trans relationship on adjacent carbons of the cyclohexane ring).
