Question
Question: The given starting material is a bicyclic compound, a decalin derivative, with four cyano (-CN) grou...
The given starting material is a bicyclic compound, a decalin derivative, with four cyano (-CN) groups attached to two adjacent carbons. One of the rings contains a double bond. The reaction conditions are H₃O⁺/Δ, which signifies acidic hydrolysis with heating. What is the final product?
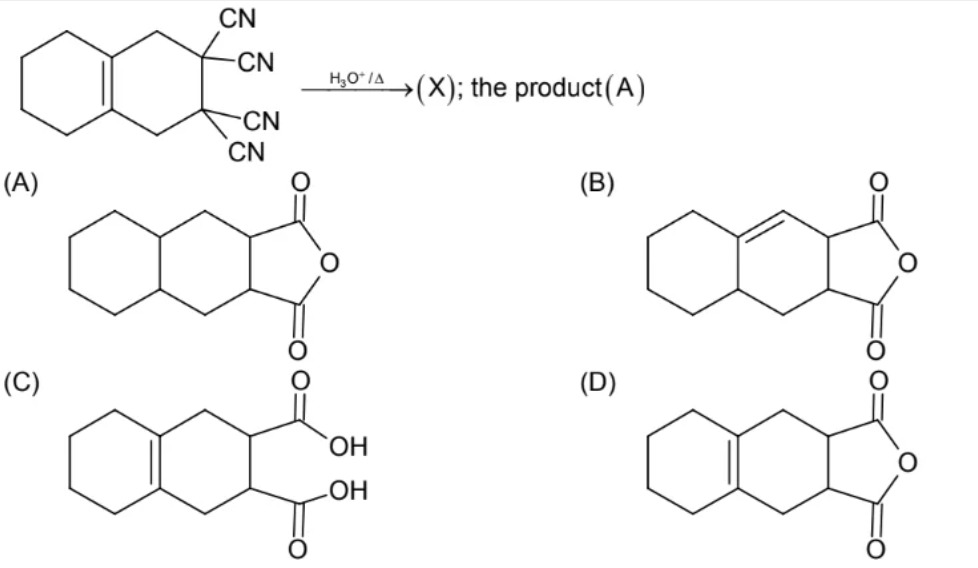
Structure A
Structure B
Structure C
Structure D
D
Solution
The reaction involves acidic hydrolysis of nitriles followed by heating. Each geminal dicyano group (-C(CN)₂) hydrolyzes to a geminal dicarboxylic acid (-C(COOH)₂). Geminal dicarboxylic acids are unstable to heat and undergo decarboxylation to form monocarboxylic acids (-CH(COOH)). Since there are two adjacent geminal dicyano groups, the initial product would be a 1,1,2,2-tetracarboxylic acid. This intermediate then undergoes two decarboxylations to yield a 1,2-dicarboxylic acid. Finally, heating a 1,2-dicarboxylic acid leads to intramolecular dehydration, forming a cyclic anhydride. The double bond in the other ring remains unaffected. The final product is structure D.
