Question
Question: The problem involves a two-step reaction sequence starting from cyclopentene. **Step 1: Reaction of...
The problem involves a two-step reaction sequence starting from cyclopentene.
Step 1: Reaction of Cyclopentene with Br₂
Cyclopentene is an alkene. Alkenes undergo electrophilic addition with halogens like bromine (Br₂). This reaction is typically an anti-addition, meaning the two bromine atoms add to opposite faces of the double bond.
The product (A) formed is 1,2-dibromocyclopentane.
-
Structure of Cyclopentene: SMILES:
C1C=CCC1CH₂-CH₂ / \ CH=CH CH₂ -
Structure of A (1,2-dibromocyclopentane): SMILES:
BrC1C(Br)CCC1CH₂-CH₂ / \ CHBr-CHBr CH₂
Step 2: Reaction of A (1,2-dibromocyclopentane) with Alcoholic KOH
Alcoholic KOH is a strong base and is used for dehydrohalogenation (elimination reaction). When a vicinal dihalide (like 1,2-dibromocyclopentane) is treated with alcoholic KOH, it undergoes double elimination of HBr to form an alkyne.
The reaction proceeds in two steps:
-
First elimination: One molecule of HBr is removed from 1,2-dibromocyclopentane to form a bromoalkene (specifically, 1-bromocyclopentene).
1,2-dibromocyclopentane + alc. KOH → 1-bromocyclopentene + KBr + H₂O -
Second elimination: Another molecule of HBr is removed from 1-bromocyclopentene to form a cycloalkyne.
1-bromocyclopentene + alc. KOH → Cyclopentyne (B) + KBr + H₂O
- Structure of B (Cyclopentyne):
SMILES:
C1#CCCC1CH₂-CH₂ / \ C≡C CH₂
Stability of Cyclopentyne:
Cyclopentyne is a highly strained molecule. The ideal bond angle for a carbon-carbon triple bond is 180°, which is impossible to achieve in a five-membered ring without significant angle strain and ring strain. Therefore, cyclopentyne is extremely unstable and cannot be isolated under normal conditions; it is typically observed only as a transient intermediate or polymerizes rapidly. However, in the context of predicting reaction products in organic chemistry, it is the theoretical product of this double dehydrohalogenation.
Summary of Products:
- A: 1,2-dibromocyclopentane
- B: Cyclopentyne
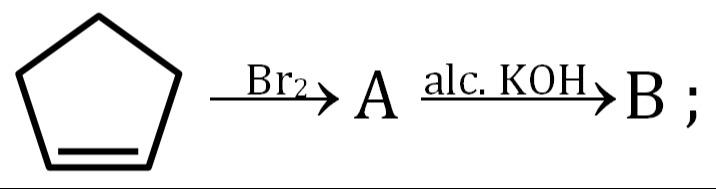
A is 1,2-dibromocyclopentane. Smiles: BrC1C(Br)CCC1
B is cyclopentyne. Smiles: C1#CCCC1
Solution
-
Cyclopentene undergoes electrophilic addition with Br₂ to form 1,2-dibromocyclopentane (A).
-
1,2-dibromocyclopentane (A) undergoes double dehydrohalogenation with alcoholic KOH. The first elimination forms 1-bromocyclopentene, and the second elimination forms cyclopentyne (B).
