Question
Question: \[Figure]\...
[Figure]\
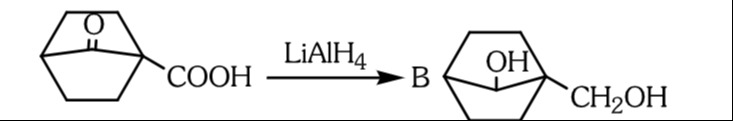
The product B shown in the reaction is correctly formed.
Solution
The given reaction involves the reduction of a bicyclic compound containing two functional groups: a ketone and a carboxylic acid. The reducing agent used is Lithium Aluminium Hydride (LiAlH₄).
Understanding the Reactant:
The starting material is a bicyclo[2.2.1]heptane derivative.
- It has a ketone group (C=O) at the bridge (C7 position).
- It has a carboxylic acid group (-COOH) attached to one of the bridgehead carbons (C1 position).
The reactant can be named as 1-carboxy-bicyclo[2.2.1]heptan-7-one.
Reactivity of LiAlH₄:
Lithium Aluminium Hydride (LiAlH₄) is a powerful reducing agent that can reduce various functional groups:
- Ketones: LiAlH₄ reduces ketones (R₂C=O) to secondary alcohols (R₂CH-OH).
- Carboxylic Acids: LiAlH₄ reduces carboxylic acids (R-COOH) to primary alcohols (R-CH₂OH).
Applying the Reaction to the Reactant:
Based on the reactivity of LiAlH₄:
- The ketone group (C=O) at the C7 position will be reduced to a secondary alcohol (-CH-OH). The oxygen atom of the ketone will gain a hydrogen and the carbon atom will gain a hydrogen.
- The carboxylic acid group (-COOH) at the C1 position will be reduced to a primary alcohol (-CH₂OH). The carbonyl carbon of the carboxylic acid will be fully reduced to a methylene group, and the hydroxyl group will remain as part of the primary alcohol.
Analyzing Product B:
The product B shown in the image is:
- The ketone bridge (C=O) has been converted to a hydroxyl group (-OH) attached to the bridge carbon, indicating the formation of a secondary alcohol.
- The carboxylic acid group (-COOH) attached to the bridgehead carbon has been converted to a primary alcohol group (-CH₂OH).
Both transformations observed in product B are consistent with the known reducing capabilities of LiAlH₄. Therefore, the product B depicted is correctly formed from the given reactant under the specified reaction conditions.
The product B is 1-(hydroxymethyl)-bicyclo[2.2.1]heptan-7-ol.
Explanation of the solution:
Lithium Aluminium Hydride (LiAlH₄) is a strong reducing agent. It reduces the ketone group (C=O) in the bicyclic system to a secondary alcohol (-CH-OH) and the carboxylic acid group (-COOH) to a primary alcohol (-CH₂OH). The product B shown in the reaction scheme correctly depicts these transformations.
