Question
Question: The image displays a 1,4-dicyanobenzene molecule (terephthalonitrile) with partial charges and elect...
The image displays a 1,4-dicyanobenzene molecule (terephthalonitrile) with partial charges and electron flow indicated. Analyze the information provided in the figure, including the electron-withdrawing nature of the cyano group, partial charges within the C≡N bond, and the inequality |δ'-| > |δ-|. Explain any inconsistencies and the core concepts illustrated.
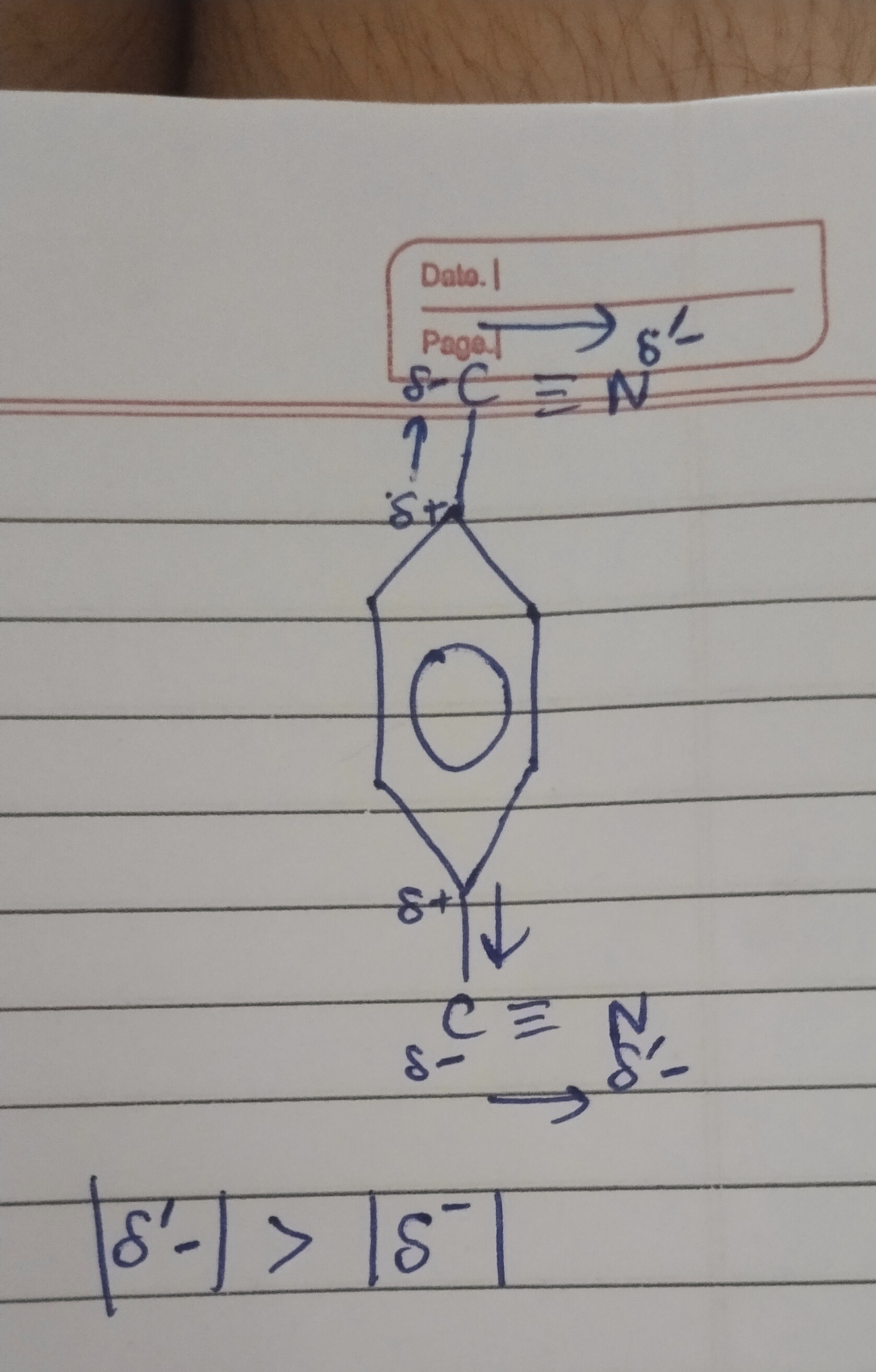
The figure illustrates the electron-withdrawing nature of the cyano group from the benzene ring. Within the C≡N bond, nitrogen is more electronegative than carbon, leading to a shift of electron density towards nitrogen. This results in nitrogen acquiring a larger partial negative charge (δ'-) compared to carbon. While the carbon of the cyano group should ideally be partially positive (δ+), the inequality |δ'-| > |δ-| correctly emphasizes that nitrogen, being more electronegative, bears a greater magnitude of partial charge compared to carbon.
Solution
The cyano group (-C≡N) is an electron-withdrawing group, pulling electron density from the benzene ring, hence the δ+ on the ring carbon. Within the C≡N bond, nitrogen is more electronegative than carbon, causing electron density to shift towards nitrogen. This makes nitrogen partially negative (δ'-) and carbon partially positive (δ+). The figure incorrectly labels the carbon as δ-. However, the inequality |δ'-| > |δ-| correctly reflects that the more electronegative nitrogen atom accumulates a greater magnitude of partial negative charge compared to the carbon atom in the C≡N bond.
