Question
Question: Options ...
Options
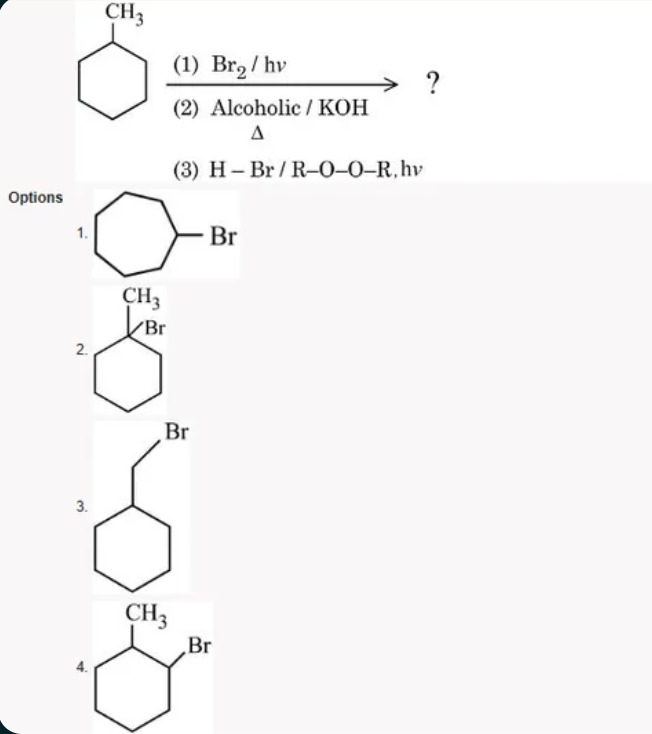
CH3 CH3 Br (1) Br2/hv (2) Alcoholic / KOH Δ (3) H-Br/R-O-O-R, hv Br
Option 4
Solution
Solution Explanation
-
Step 1 (Radical Bromination):
In methylcyclohexane, Br₂/hν preferentially brominates a secondary hydrogen. The most favored site is on the ring carbon already bearing the –CH₃ group (better than the primary hydrogens of –CH₃). This yields a bromo derivative with –CH₃ and Br on the same carbon. -
Step 2 (Elimination):
Treatment with alcoholic KOH and heat causes an E2 elimination (loss of HBr) to form an alkene. The most likely alkene is 1‑methylcyclohexene (double bond between the substituted carbon and an adjacent ring carbon). -
Step 3 (Anti-Markovnikov HBr Addition):
Adding HBr in the presence of a peroxide (R–O–O–R, hv) proceeds via a free radical mechanism (anti‑Markovnikov addition). The Br attaches to the less substituted carbon of the double bond. For 1‑methylcyclohexene, Br adds to the carbon adjacent to the one bearing –CH₃. -
Final Product:
The net transformation results in a cyclohexane ring with –CH₃ on one carbon and –Br on an adjacent carbon.
Thus, the correct option is 4.
