Question
Question: [Figure] ...
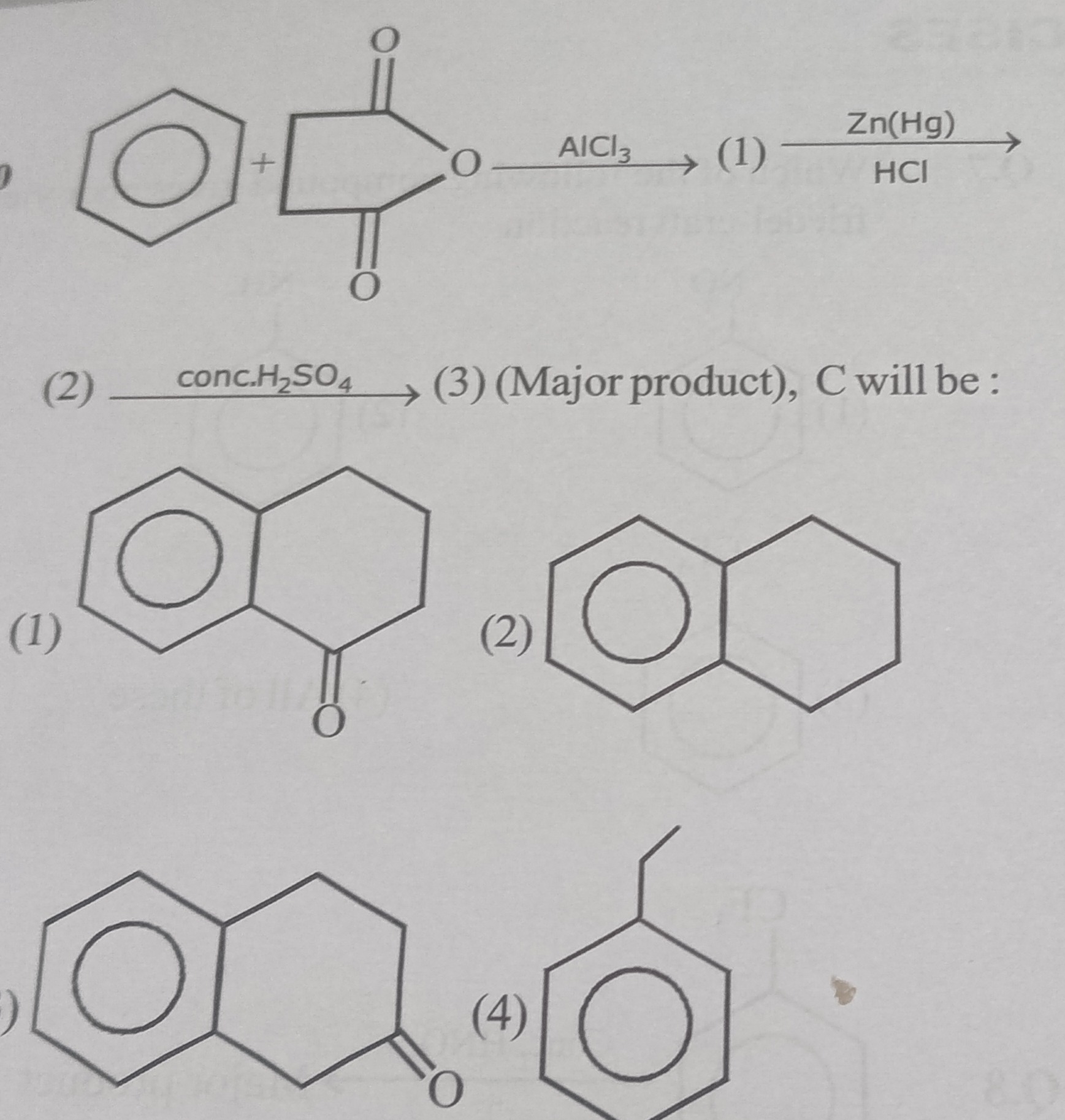
[Figure]
1
Solution
The problem involves a sequence of organic reactions starting from benzene and succinic anhydride.
Step 1: Friedel-Crafts Acylation
The first reaction involves benzene reacting with succinic anhydride in the presence of AlCl₃. This is a Friedel-Crafts acylation. Succinic anhydride acts as an acylating agent. One of the carbonyl groups of succinic anhydride reacts with benzene, while the other carbonyl group is hydrolyzed to a carboxylic acid.
The product (1) formed is 4-oxo-4-phenylbutanoic acid (also known as β-benzoylpropionic acid).
Structure of Product (1):
O=C(c1ccccc1)CCC(=O)OH
Step 2: Clemmensen Reduction
Product (1), 4-oxo-4-phenylbutanoic acid, is then subjected to Clemmensen reduction using Zn(Hg)/HCl. Clemmensen reduction is used to reduce a ketone or aldehyde to an alkane (methylene group, -CH₂-). The carboxylic acid group (-COOH) is generally resistant to Clemmensen reduction.
The keto group (-C=O) in 4-oxo-4-phenylbutanoic acid is reduced to a methylene group (-CH₂-).
The product (2) formed is 4-phenylbutanoic acid.
Structure of Product (2):
CH2(c1ccccc1)CH2CH2C(=O)OH
Step 3: Intramolecular Friedel-Crafts Acylation (Cyclization)
Product (2), 4-phenylbutanoic acid, is treated with concentrated H₂SO₄. Concentrated H₂SO₄ acts as a strong acid and dehydrating agent, facilitating intramolecular Friedel-Crafts acylation. The carboxylic acid group is activated (e.g., by protonation and loss of water to form an acylium ion or an activated acyl species), and the benzene ring undergoes an electrophilic attack on the carbonyl carbon. This leads to the formation of a stable cyclic ketone.
For 4-phenylbutanoic acid (C6H5-CH2-CH2-CH2-COOH), the benzene ring can attack the carbonyl carbon of the carboxylic acid group, forming a 6-membered ring. This cyclization reaction produces 1-tetralone.
Structure of Product (3) (C):
O=C1CCCC2=C1C=CC=C2 (1-tetralone)
Now, let's compare this structure with the given options:
- Option (1) shows 1-tetralone (a benzene ring fused with a cyclohexanone ring where the carbonyl group is adjacent to the fusion point). This matches our derived product (3).
- Option (2) shows tetralin (a benzene ring fused with a saturated cyclohexane ring).
- Option (3) shows 2-tetralone (a benzene ring fused with a cyclohexanone ring where the carbonyl group is at position 2 relative to the fusion point).
- Option (4) shows ethylbenzene.
Therefore, the major product C is 1-tetralone, which corresponds to option (1).
The complete reaction sequence is:
- Benzene + Succinic anhydride AlCl3 4-oxo-4-phenylbutanoic acid (Product 1)
- 4-oxo-4-phenylbutanoic acid Zn(Hg)/HCl 4-phenylbutanoic acid (Product 2)
- 4-phenylbutanoic acid conc. H2SO4 1-tetralone (Product 3, C)
The final answer is 1
Explanation (minimal):
- Benzene reacts with succinic anhydride via Friedel-Crafts acylation to form 4-oxo-4-phenylbutanoic acid.
- Clemmensen reduction (Zn(Hg)/HCl) reduces the ketone in 4-oxo-4-phenylbutanoic acid to a methylene group, yielding 4-phenylbutanoic acid.
- Intramolecular Friedel-Crafts acylation of 4-phenylbutanoic acid with conc. H₂SO₄ leads to cyclization, forming a stable 6-membered cyclic ketone, 1-tetralone. This matches option (1).
