Question
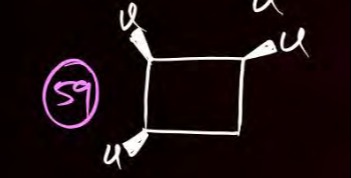
Question: The given image displays a chemical structure, which is a cyclobutane ring with four chlorine atoms ...
The given image displays a chemical structure, which is a cyclobutane ring with four chlorine atoms attached. The representation uses wedges and dashes to indicate the stereochemistry of the substituents.
Let's analyze the stereochemistry at each carbon atom of the cyclobutane ring:
- Let's label the carbons C1 (top right), C2 (top left), C3 (bottom left), and C4 (bottom right).
- At C1: The chlorine atom is on a wedge (coming out of the plane), and the hydrogen atom (implied) is on a dash (going into the plane). We can denote this as Cl(up), H(down).
- At C2: The chlorine atom is on a dash (going into the plane), and the hydrogen atom is on a wedge (coming out of the plane). We can denote this as Cl(down), H(up).
- At C3: The chlorine atom is on a wedge (coming out of the plane), and the hydrogen atom is on a dash (going into the plane). We can denote this as Cl(up), H(down).
- At C4: The chlorine atom is on a dash (going into the plane), and the hydrogen atom is on a wedge (coming out of the plane). We can denote this as Cl(down), H(up).
This arrangement indicates that adjacent chlorine atoms are trans to each other (e.g., Cl on C1 is 'up', Cl on C2 is 'down'). This is therefore all-trans-1,2,3,4-tetrachlorocyclobutane.
To determine if the molecule is chiral (optically active) or achiral (optically inactive), we first check for the presence of chiral centers and then for symmetry elements.
1. Chiral Centers:
A carbon atom is a chiral center if it is bonded to four different groups. In a cyclic compound, the two paths around the ring from a given carbon must be different for that carbon to be considered a chiral center.
Let's examine C1:
- Group 1: Cl (wedge)
- Group 2: H (dash)
- Group 3: The ring path C1-C2-C3-C4
- Group 4: The ring path C1-C4-C3-C2
To determine if Group 3 and Group 4 are different, we trace along the ring in both directions, noting the atoms and their stereochemistry:
- Path C1-C2-C3-C4: From C1(Cl_up), we go to C2(Cl_down), then C3(Cl_up), then C4(Cl_down).
- Path C1-C4-C3-C2: From C1(Cl_up), we go to C4(Cl_down), then C3(Cl_up), then C2(Cl_down).
Comparing the two paths:
- The first carbon encountered from C1 in both paths (C2 and C4, respectively) has the same stereochemistry (Cl_down, H_up).
- The second carbon encountered (C3 in both paths) has the same stereochemistry (Cl_up, H_down).
- The third carbon encountered (C4 in the first path, C2 in the second path) has the same stereochemistry (Cl_down, H_up).
Since the sequence of atoms and their stereochemistry is identical in both directions around the ring from C1, the two ring paths attached to C1 are identical. Therefore, C1 is not a chiral center.
By symmetry, C2, C3, and C4 are also not chiral centers.
Conclusion on Chiral Centers: The molecule has no chiral centers.
2. Symmetry Elements:
A molecule is achiral if it possesses a plane of symmetry (σ) or a center of inversion (i).
Let's consider a plane passing through C1 and C3, bisecting the C2-C4 bond.
- This plane contains C1 and C3.
- C1 and C3 both have Cl(up) and H(down).
- C2 and C4 are outside this plane. C2 has Cl(down), H(up). C4 has Cl(down), H(up).
- If we reflect C2 (with its substituents) across this plane, it maps onto C4 (with its substituents).
- Therefore, there is a plane of symmetry passing through C1 and C3.
- Similarly, there is a plane of symmetry passing through C2 and C4.
Conclusion on Symmetry: The molecule possesses a plane of symmetry.
Achiral and optically inactive
Solution
The molecule is all-trans-1,2,3,4-tetrachlorocyclobutane. Each carbon atom in the ring is bonded to a chlorine, a hydrogen, and two ring carbons. Upon tracing the ring paths from any carbon in both directions, the sequence of atoms and their stereochemistry is found to be identical. This means none of the carbon atoms are chiral centers. Furthermore, the molecule possesses a plane of symmetry (e.g., passing through C1 and C3). Since it has no chiral centers and possesses a plane of symmetry, the molecule is achiral and thus optically inactive.
