Question
Question: HBr $\longrightarrow$...
HBr ⟶
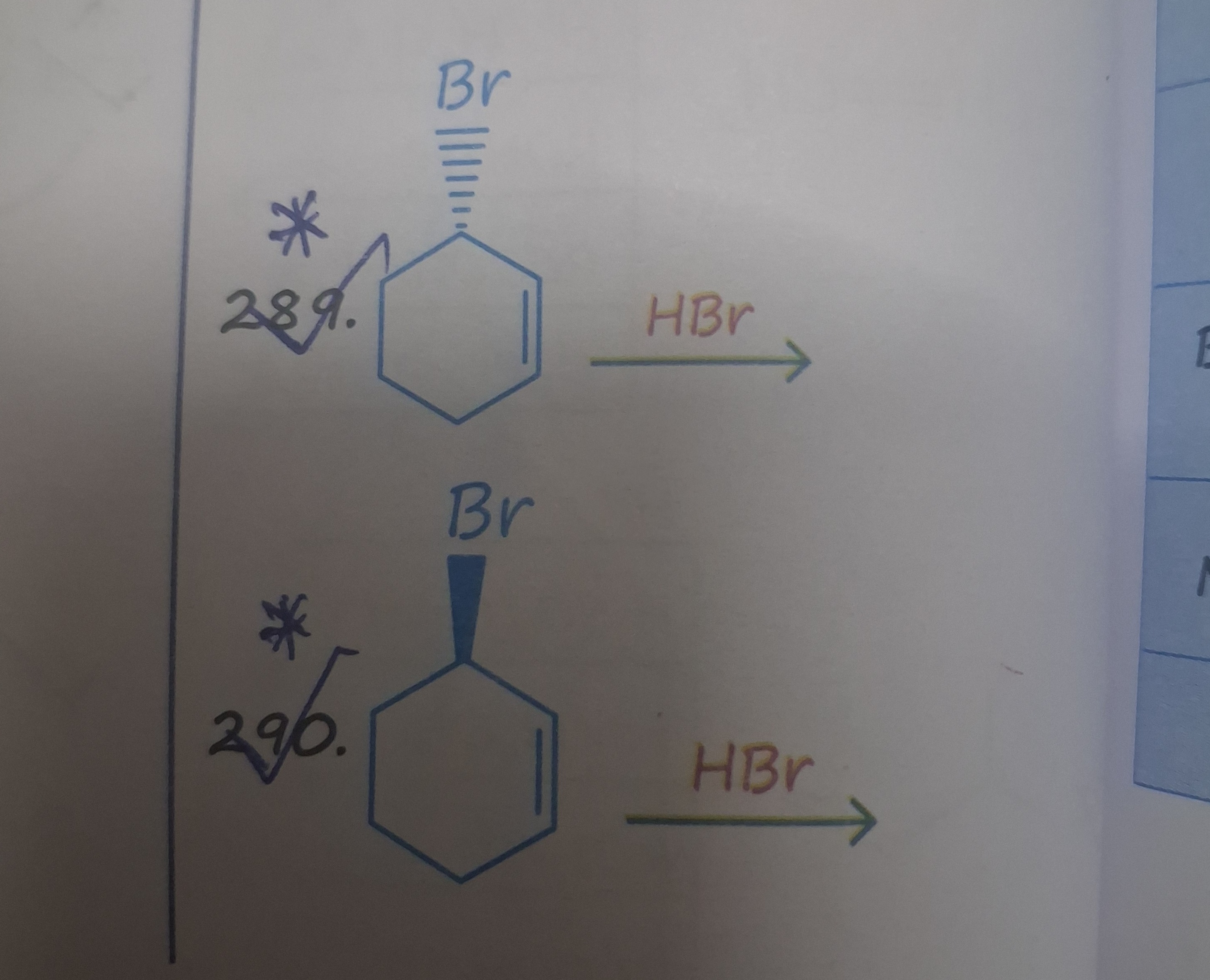
A
cis-1,3-dibromocyclohexane
B
trans-1,3-dibromocyclohexane
C
1,2-dibromocyclohexane
D
1,4-dibromocyclohexane
Answer
trans-1,3-dibromocyclohexane
Explanation
Solution
The reaction is an electrophilic addition of HBr to 3-bromocyclohexene. H+ adds to the double bond to form the more stable carbocation, which is at C1 (adjacent to the double bond and further from the electron-withdrawing bromine at C3). Br- then attacks the carbocation at C1. Due to steric hindrance from the bromine at C3, the attack of Br- is favored from the face opposite to the existing bromine, leading to the trans product as the major stereoisomer in both cases (for questions 289 and 290, which differ only in the stereochemistry of the initial bromine substituent).
