Question
Question: ...
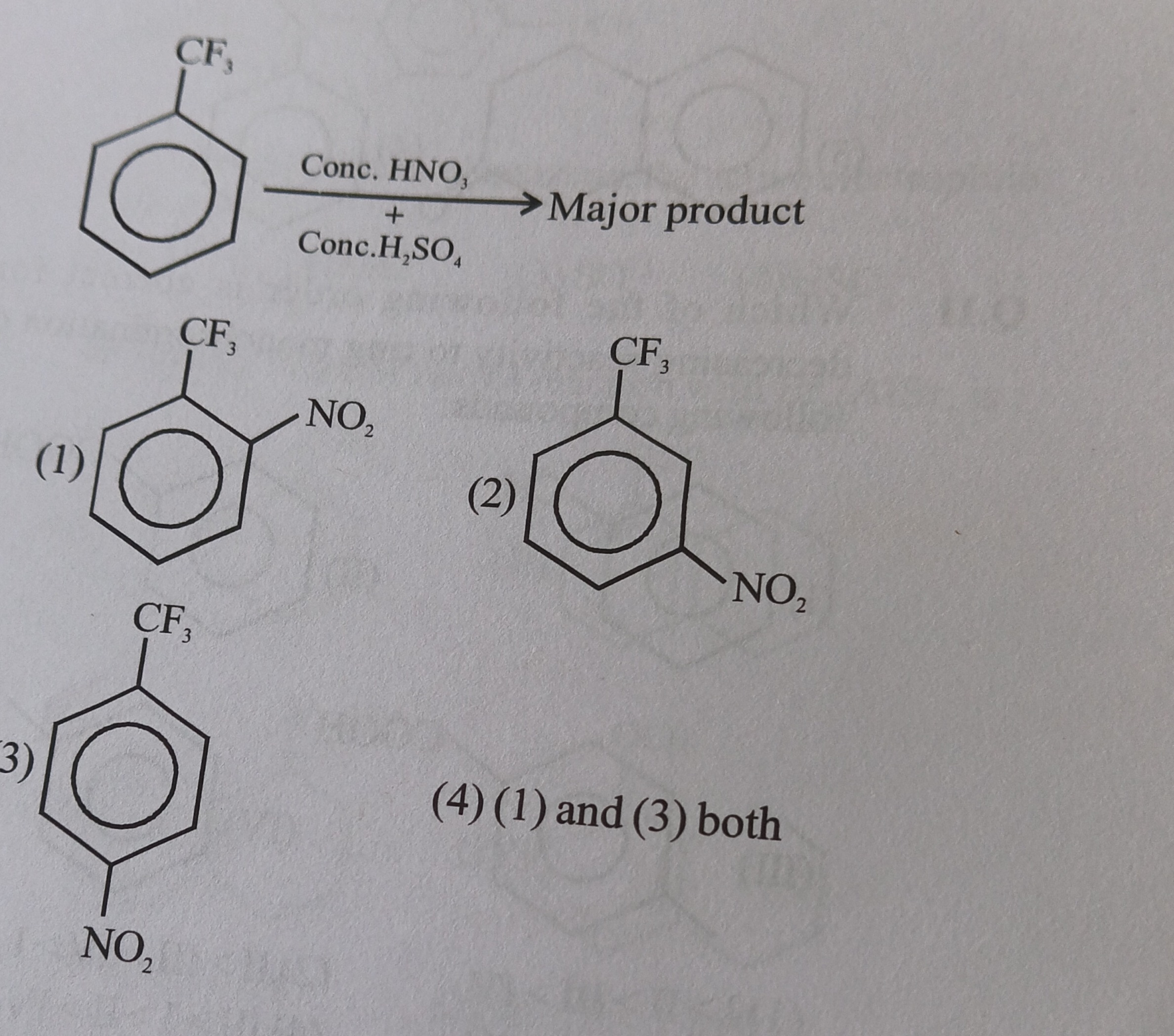
2
Solution
The reaction shown is nitration of trifluoromethylbenzene with concentrated nitric acid and concentrated sulfuric acid. This is an electrophilic aromatic substitution reaction.
1. Identify the Electrophile: In the nitration reaction, concentrated HNO₃ and concentrated H₂SO₄ react to generate the nitronium ion (NO₂⁺), which is the electrophile. HNO3+2H2SO4⇌NO2++H3O++2HSO4−
2. Analyze the Directing Effect of the -CF₃ Group: The trifluoromethyl (-CF₃) group is attached to the benzene ring. To determine its directing effect, we need to consider its electronic effects:
- Inductive Effect (-I effect): Fluorine is the most electronegative element. The three fluorine atoms in the -CF₃ group strongly withdraw electron density from the carbon atom to which they are attached. This carbon, in turn, withdraws electron density from the benzene ring through the sigma bond. Therefore, the -CF₃ group exhibits a strong electron-withdrawing inductive effect (-I effect).
- Resonance Effect (Mesomeric Effect): The -CF₃ group does not have lone pairs or pi bonds that can conjugate with the benzene ring. Thus, it does not exert any significant mesomeric effect.
Since the -CF₃ group is strongly electron-withdrawing primarily due to its -I effect, it deactivates the benzene ring towards electrophilic aromatic substitution. Electron-withdrawing groups are generally meta-directing.
3. Justification for Meta-Directing Nature: During electrophilic aromatic substitution, a carbocation intermediate (Wheland intermediate) is formed. The stability of this intermediate determines the regioselectivity.
- If the electrophile attacks the ortho or para positions, one of the resonance structures of the carbocation intermediate will place a positive charge directly on the carbon atom bearing the -CF₃ group. The strongly electron-withdrawing -CF₃ group will severely destabilize this positive charge, making ortho and para attacks highly unfavorable.
- If the electrophile attacks the meta position, none of the resonance structures of the carbocation intermediate place a positive charge directly on the carbon atom bearing the -CF₃ group. Although the ring is still deactivated, the meta position is comparatively less deactivated than the ortho and para positions.
Therefore, the attack at the meta position leads to a relatively more stable carbocation intermediate, making the meta product the major product.
4. Determine the Major Product: Since the -CF₃ group is meta-directing, the incoming -NO₂ group will attach to the meta position relative to the -CF₃ group.
The starting material is trifluoromethylbenzene:
The major product will be 1-trifluoromethyl-3-nitrobenzene:
This corresponds to option (2).
