Question
Question: ...
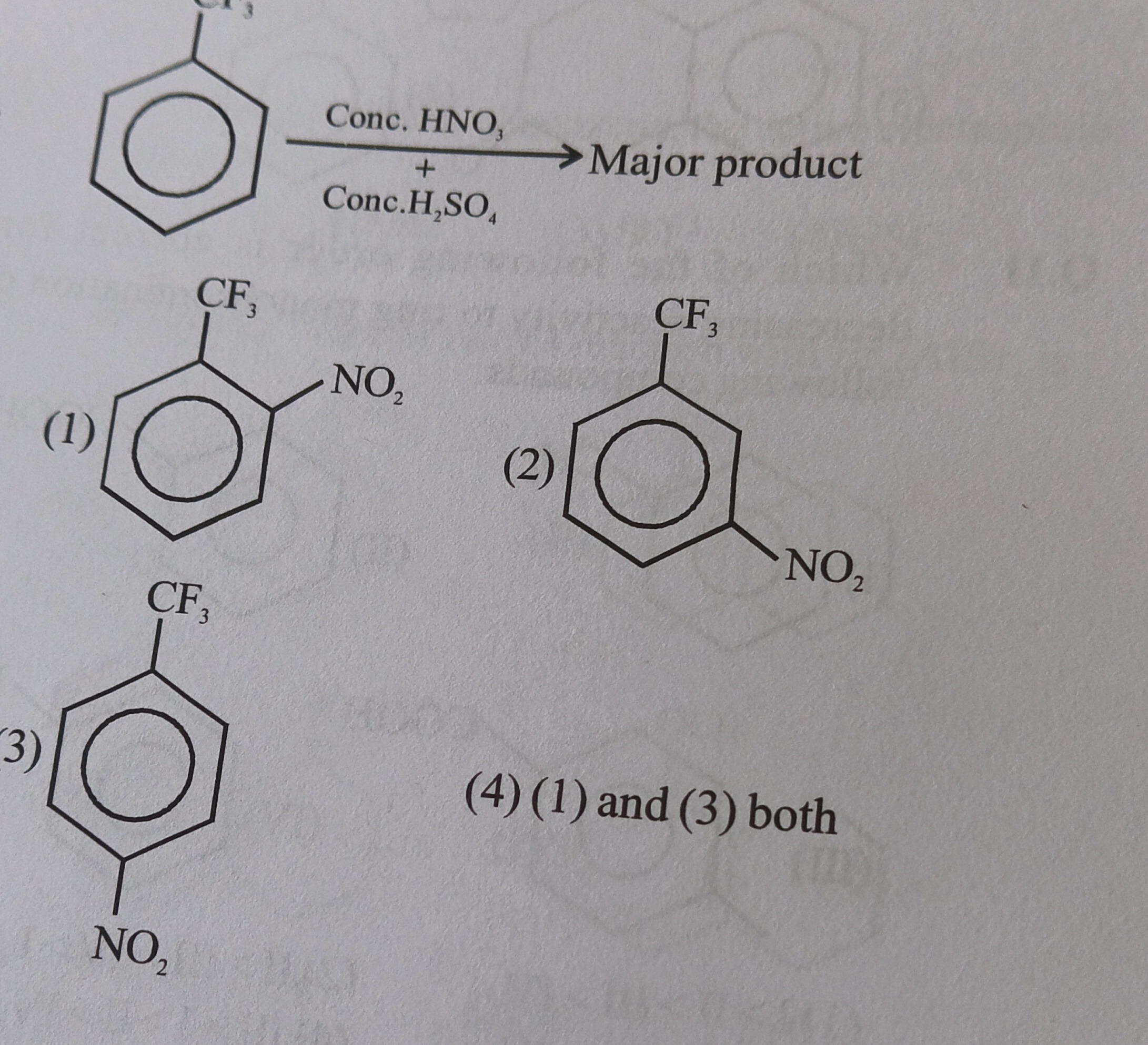
The reaction shown is nitration of trifluoromethylbenzene using a mixture of concentrated nitric acid and concentrated sulfuric acid. This is an electrophilic aromatic substitution reaction.
1. Identify the Electrophile: The mixture of concentrated HNO₃ and concentrated H₂SO₄ generates the nitronium ion (NO₂⁺), which is the electrophile for nitration.
HNO3+2H2SO4⇌NO2++H3O++2HSO4−2. Analyze the directing nature of the -CF₃ group: The -CF₃ (trifluoromethyl) group is a strong electron-withdrawing group. This is due to the high electronegativity of the fluorine atoms, which exert a strong inductive electron-withdrawing effect (-I effect) on the carbon atom to which they are attached, and this effect is transmitted to the benzene ring.
Electron-withdrawing groups are generally meta-directing and deactivating towards electrophilic aromatic substitution.
3. Explain the meta-directing effect: In electrophilic aromatic substitution, the electrophile attacks the benzene ring to form a carbocation intermediate (sigma complex). For electron-withdrawing groups:
-
Ortho and Para attack: If the electrophile attacks at the ortho or para position, one of the resonance structures of the carbocation intermediate places a positive charge directly on the carbon atom bearing the electron-withdrawing group. This is highly unfavorable because the electron-withdrawing group already makes that carbon electron-deficient and would further destabilize a positive charge at that position.
-
Meta attack: If the electrophile attacks at the meta position, the positive charge in the carbocation intermediate is never placed on the carbon atom bearing the electron-withdrawing group. This avoids the highly unstable resonance structure seen in ortho and para attacks.
Therefore, the meta carbocation intermediate is relatively more stable than the ortho or para intermediates, making meta substitution the preferred pathway.
4. Determine the major product: Since the -CF₃ group is a meta-director, the incoming nitronium ion (NO₂⁺) will preferentially attack the meta position relative to the -CF₃ group.
Let's consider the structure of trifluoromethylbenzene:
If the -CF₃ group is at position 1, the meta positions are positions 3 and 5 (which are equivalent due to symmetry).
Therefore, the NO₂ group will attach at position 3 (or 5). The major product will be 1-trifluoromethyl-3-nitrobenzene.
5. Match with options:
- Option (1) shows ortho-nitration (1-trifluoromethyl-2-nitrobenzene).
- Option (2) shows meta-nitration (1-trifluoromethyl-3-nitrobenzene).
- Option (3) shows para-nitration (1-trifluoromethyl-4-nitrobenzene).
Based on the meta-directing nature of the -CF₃ group, option (2) represents the major product.
The final answer is 2
The correct option is (2). Nitration of trifluoromethylbenzene involves electrophilic aromatic substitution. The -CF₃ group is a strong electron-withdrawing group, making it a meta-director. This is because electron-withdrawing groups destabilize the carbocation intermediates formed by ortho and para attack more than the meta attack intermediate, as ortho/para attack places a positive charge on the carbon bearing the electron-withdrawing group. Thus, the nitronium ion (NO₂⁺) attacks the meta position, yielding 1-trifluoromethyl-3-nitrobenzene as the major product. medium Chemistry Organic Chemistry - Hydrocarbons (specifically Aromatic Compounds) or Haloalkanes and Haloarenes (as CF3 is a halogenated group) Electrophilic Aromatic Substitution - Directing Effects of Substituents single_choice
Solution
Nitration of trifluoromethylbenzene involves electrophilic aromatic substitution. The -CF₃ group is a strong electron-withdrawing group, making it a meta-director. This is because electron-withdrawing groups destabilize the carbocation intermediates formed by ortho and para attack more than the meta attack intermediate, as ortho/para attack places a positive charge on the carbon bearing the electron-withdrawing group. Thus, the nitronium ion (NO₂⁺) attacks the meta position, yielding 1-trifluoromethyl-3-nitrobenzene as the major product.
