Question
Question: ...
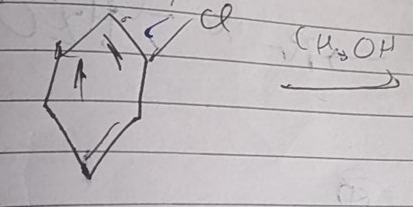
Answer
1-methoxybicyclo[2.1.1]hexane
Explanation
Solution
The reaction of 6-chlorobicyclo[3.1.0]hexane with methanol proceeds via an SN1 mechanism involving a carbocation intermediate. The initial 6-bicyclo[3.1.0]hexyl cation is a cyclopropylcarbinyl cation, which is highly prone to skeletal rearrangement. This cation rearranges to the more stable 1-bicyclo[2.1.1]hexyl cation through a Wagner-Meerwein type rearrangement involving the migration of the C1-C5 bridgehead bond to C6. The rearranged carbocation then undergoes nucleophilic attack by methanol, followed by deprotonation, to yield 1-methoxybicyclo[2.1.1]hexane as the final product.
