Question
Question: The given reaction involves benzyl chloride ($\text{C}_6\text{H}_5\text{CH}_2\text{Cl}$) reacting wi...
The given reaction involves benzyl chloride (C6H5CH2Cl) reacting with methanol (CH3OH). What is the product of this reaction?
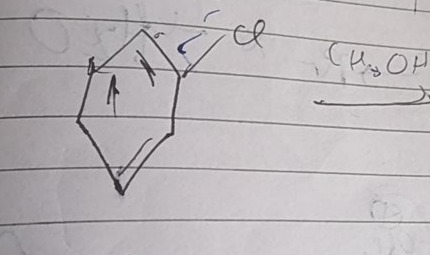
Answer
Benzyl methyl ether
Explanation
Solution
The reaction of benzyl chloride with methanol is a nucleophilic substitution reaction (SN1).
-
Formation of Benzylic Carbocation: The chlorine atom leaves as a chloride ion, forming a stable benzylic carbocation:
C6H5CH2ClSlowC6H5CH2++Cl−The benzylic carbocation is resonance-stabilized by the benzene ring.
-
Nucleophilic Attack: The oxygen atom of methanol attacks the positively charged benzylic carbon:
C6H5CH2++CH3OHFastC6H5CH2−O+(H)CH3 -
Deprotonation: The protonated ether intermediate loses a proton, yielding benzyl methyl ether:
C6H5CH2−O+(H)CH3+CH3OH⟶C6H5CH2−O−CH3+CH3OH2+
The overall reaction replaces the chlorine atom with a methoxy (-OCH3) group, forming benzyl methyl ether.
