Question
Question: ...
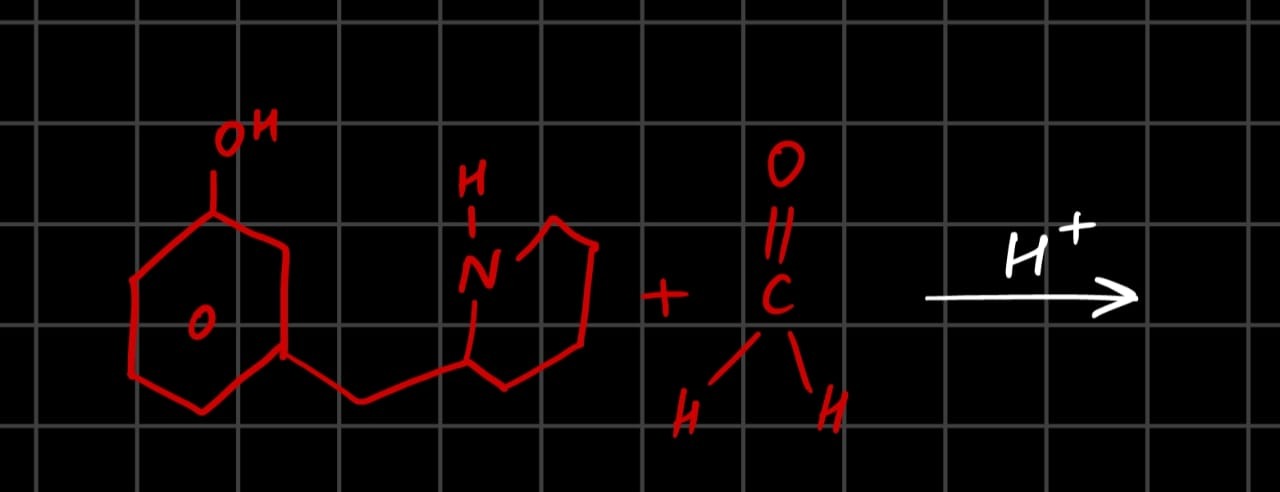
O.C1C(N(C1)CC2=C(O)C=CC=C2)
Solution
The reaction shown is an intramolecular Mannich reaction. The secondary amine (N-H in the piperidine ring) reacts with formaldehyde to form an iminium ion intermediate. This iminium ion is then attacked by the electron-rich ortho position (C6) of the phenol ring (activated by the -OH group). This leads to the formation of a new cyclic system, specifically a methylene bridge between the nitrogen of the piperidine ring and the C6 of the benzene ring.
The product is a fused tricyclic compound.
The product structure is:
This is a Mannich base formed by intramolecular cyclization.
The final product is a tricyclic compound where the piperidine ring is fused to the benzene ring via the original connection and a new methylene bridge connects the nitrogen of the piperidine ring to the ortho position (C6) of the benzene ring. The hydroxyl group remains on the benzene ring.
