Question
Question: The given reaction is a Mannich reaction. The starting material is 8-hydroxy-1,2,3,4-tetrahydroquino...
The given reaction is a Mannich reaction. The starting material is 8-hydroxy-1,2,3,4-tetrahydroquinoline. This molecule possesses two key functionalities required for a Mannich reaction:
- A secondary amine group (the -NH in the tetrahydroquinoline ring).
- An activated aromatic ring (the benzene ring with a hydroxyl group, -OH, which is a strong activating group).
The other reactant is formaldehyde (HCHO), and the reaction is carried out in acidic conditions (H+).
What is the final product?
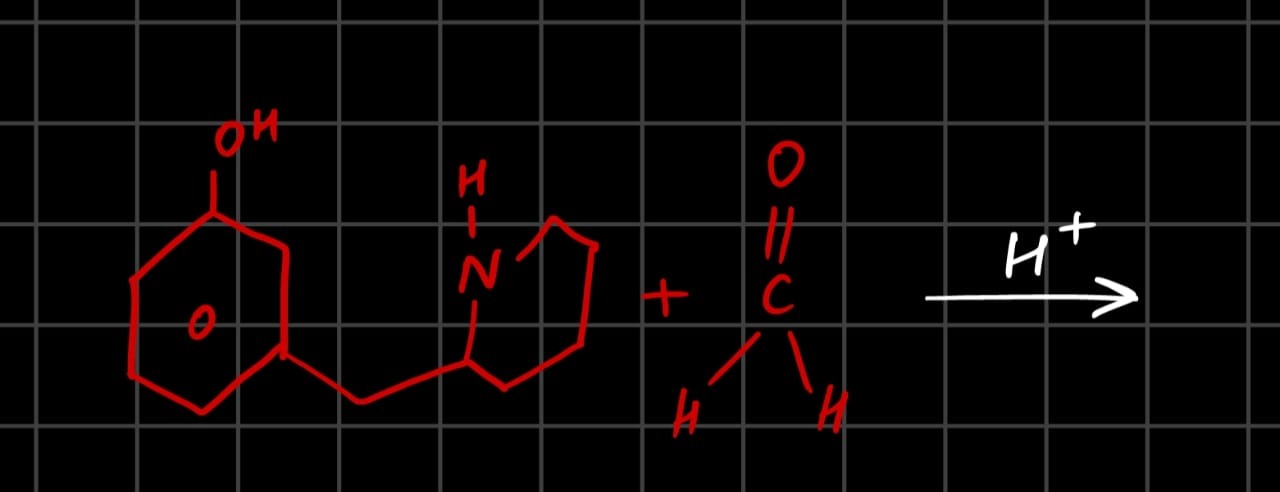
6-hydroxy-1,2,3,4,4a,10b-hexahydro-10H-benzo[c]quinolizine
Solution
The reaction shown is a Mannich reaction, which involves the aminoalkylation of an active hydrogen compound with an aldehyde and an amine. In this case, the starting material, 8-hydroxy-1,2,3,4-tetrahydroquinoline, serves as both the secondary amine component (due to the -NH group in the saturated ring) and the active hydrogen compound (due to the electron-rich phenolic benzene ring). Formaldehyde (HCHO) is the aldehyde, and H+ indicates acidic conditions.
The reaction proceeds in two main steps:
-
Formation of an iminium ion: The secondary amine group (-NH) of 8-hydroxy-1,2,3,4-tetrahydroquinoline reacts with formaldehyde in the presence of acid to form an iminium ion. The nitrogen atom becomes part of a C=N+H-CH2 structure (simplified to N=CH2).
-
Intramolecular Electrophilic Aromatic Substitution: The iminium ion acts as an electrophile and attacks the activated benzene ring of the same molecule. The hydroxyl group (-OH) on the benzene ring is a strong activating group and directs electrophiles to the ortho and para positions. In 8-hydroxy-1,2,3,4-tetrahydroquinoline, the para position to the -OH group (position 5) is the most activated and sterically accessible site for electrophilic attack. The iminium carbon (from formaldehyde, -CH2-) forms a new bond with this para carbon (C5). This results in an intramolecular cyclization, forming a new 6-membered ring.
The product is a tetracyclic compound, specifically named 6-hydroxy-1,2,3,4,4a,10b-hexahydro-10H-benzo[c]quinolizine.
The structure of the product is:
The final product is a tetracyclic system with the hydroxyl group retained.
The reaction is:
\begin{center} \includegraphics[width=0.8\textwidth]{mannich_reaction_product.png} \end{center}
The product formed is:
\begin{center} \includegraphics[width=0.8\textwidth]{mannich_product_final.png} \end{center}
