Question
Question: ...
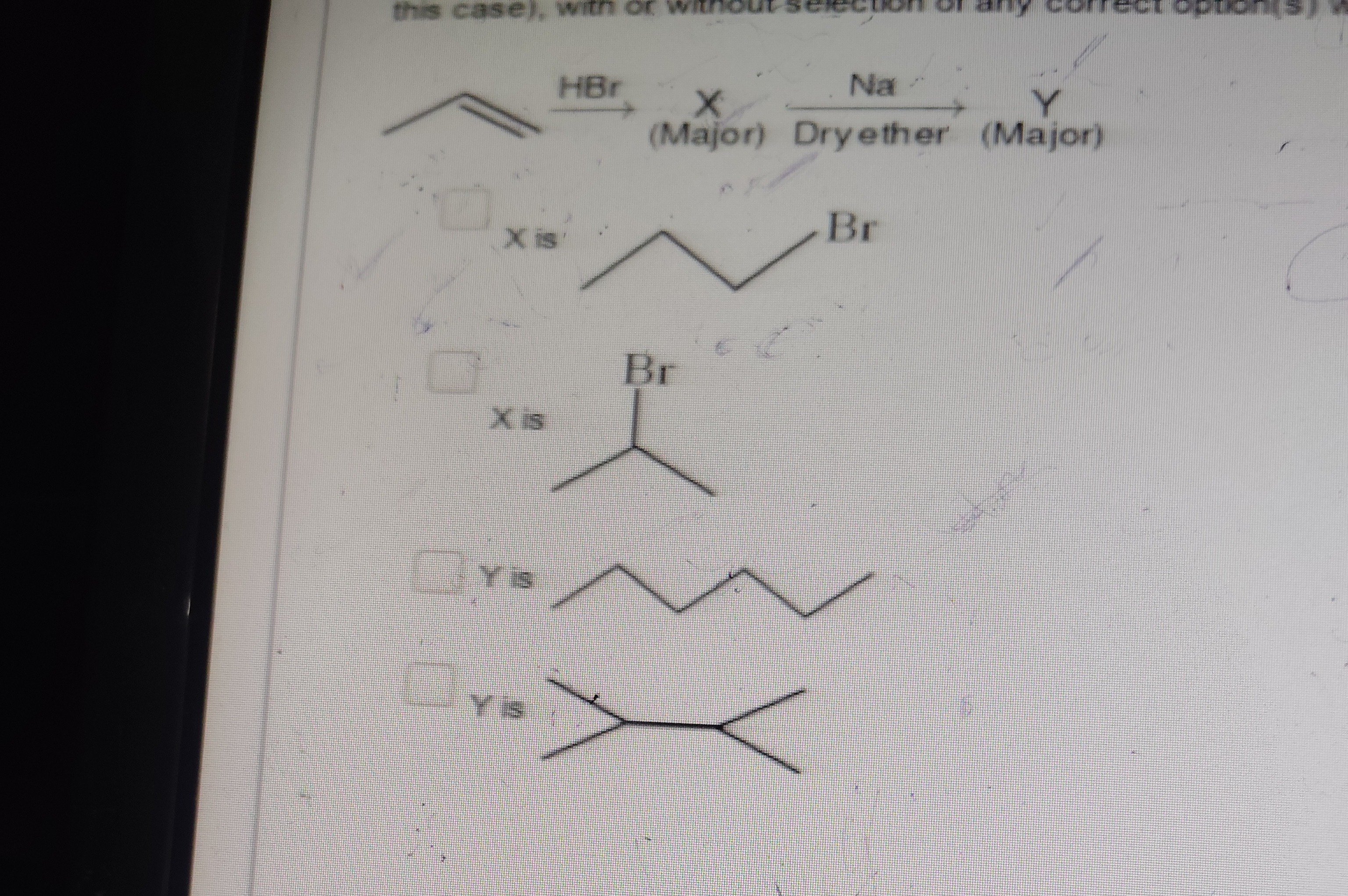
X is 2-bromobutane.
X is 2-bromopropane.
Y is hexane.
Y is 2,3-dimethylbutane.
2, 4
Solution
The reaction sequence involves two steps.
Step 1: Reaction of propene with HBr.
Propene is CH3CH=CH2. The addition of HBr to propene follows Markovnikov's rule, where the hydrogen adds to the carbon with more hydrogens, and the bromine adds to the more substituted carbon. This occurs via the formation of the more stable secondary carbocation.
CH3CH=CH2 + HBr → CH3CH+CH3 + Br− → CH3CH(Br)CH3
The major product X is 2-bromopropane.
Step 2: Reaction of X with Na in dry ether.
This is the Wurtz reaction. The Wurtz reaction involves the coupling of two molecules of an alkyl halide in the presence of sodium metal in dry ether to form a higher alkane.
2 R-X + 2 Na dry ether R-R + 2 NaX
Here, X is 2-bromopropane (CH3CH(Br)CH3).
2 CH3CH(Br)CH3 + 2 Na dry ether CH3CH(CH3)-CH(CH3)CH3 + 2 NaBr
The major product Y is 2,3-dimethylbutane.
Therefore, the correct options are those that correctly identify X and Y.
