Question
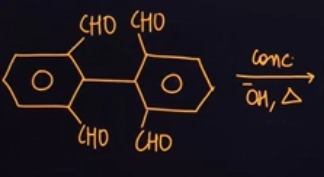
Question: The given reaction is the Cannizzaro reaction of 2,2',6,6'-tetraformylbiphenyl. This molecule has fo...
The given reaction is the Cannizzaro reaction of 2,2',6,6'-tetraformylbiphenyl. This molecule has four aldehyde groups, and each aldehyde group is attached to a carbon atom of the benzene ring that does not have any α-hydrogen atoms. The reaction conditions (concentrated OH− and heat) are suitable for the Cannizzaro reaction.
The molecule has two sets of ortho aldehyde groups, one on each benzene ring (at positions 2 and 6 on the left ring, and at positions 2' and 6' on the right ring). Intramolecular Cannizzaro reaction is expected to occur between the ortho aldehyde groups on each ring.
In an intramolecular Cannizzaro reaction between two aldehyde groups, one aldehyde group is reduced to a primary alcohol (-CH2OH) and the other is oxidized to a carboxylic acid salt (-COO−).
So, on the left benzene ring, the two aldehyde groups at positions 2 and 6 will undergo intramolecular Cannizzaro reaction. One will become -CH2OH and the other will become -COO−. Due to symmetry, the product will be a mixture of isomers where the -CH2OH is at position 2 and -COO− is at position 6, and vice versa. However, since the reaction occurs on both rings simultaneously, and the molecule has symmetry, the resulting product will have a -CH2OH and a -COO− group on each ring at the ortho positions.
Let's assume that on the left ring, the aldehyde at position 2 is reduced to -CH2OH and the aldehyde at position 6 is oxidized to -COO−. On the right ring, similarly, the aldehyde at position 2' is reduced to -CH2OH and the aldehyde at position 6' is oxidized to -COO−. The product formed is 2,2'-bis(hydroxymethyl)biphenyl-6,6'-dicarboxylate.
Alternatively, on the left ring, the aldehyde at position 2 is oxidized to -COO− and the aldehyde at position 6 is reduced to -CH2OH. On the right ring, the aldehyde at position 2' is oxidized to -COO− and the aldehyde at position 6' is reduced to -CH2OH. The product formed is 6,6'-bis(hydroxymethyl)biphenyl-2,2'-dicarboxylate.
Due to the symmetry of the starting material, these two products are equivalent. The product has two -CH2OH groups and two -COO− groups, with one of each on each ring at the ortho positions.
2,2'-bis(hydroxymethyl)biphenyl-6,6'-dicarboxylate
Solution
The given molecule is 2,2',6,6'-tetraformylbiphenyl, which has four aldehyde groups without α-hydrogens. Under concentrated base and heat, it undergoes the Cannizzaro reaction. Since there are ortho aldehyde groups on each ring, intramolecular Cannizzaro reaction occurs on each ring. In this reaction, one aldehyde group is reduced to a primary alcohol (-CH2OH) and the other is oxidized to a carboxylate salt (-COO−). Thus, the product has two -CH2OH groups and two -COO− groups. For example, the aldehyde groups at positions 2 and 2' are reduced, and the aldehyde groups at positions 6 and 6' are oxidized, resulting in 2,2'-bis(hydroxymethyl)biphenyl-6,6'-dicarboxylate.
