Question
Question: Figure...
Figure
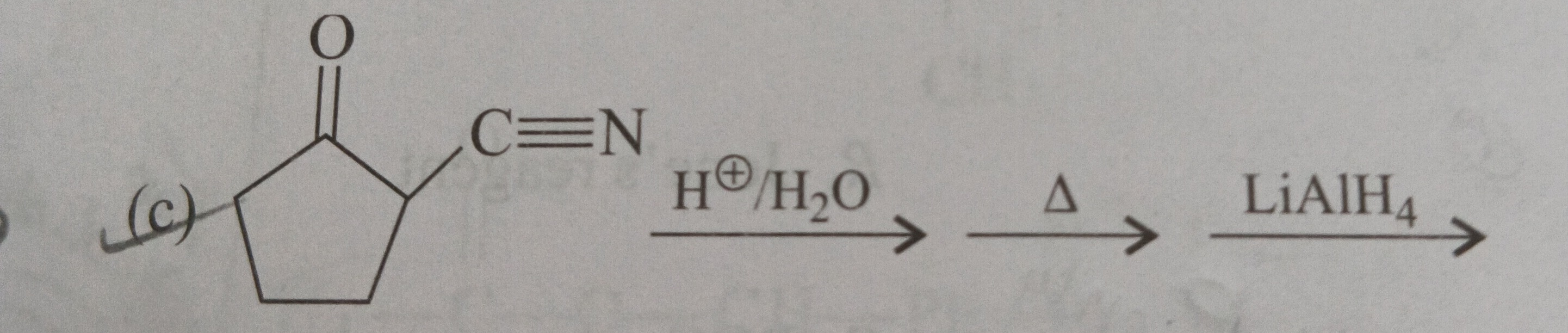
2-methylcyclopentan-1-ol
Solution
The reaction sequence starts with 2-(cyanomethyl)cyclopentan-1-one.
Step 1: Acidic hydrolysis of the nitrile group (-CN) under heating (H+/H2O,Δ).
Nitriles are hydrolyzed to carboxylic acids under acidic conditions with heating.
The −CH2CN group is converted to a −CH2COOH group.
The intermediate product is 2-(carboxymethyl)cyclopentan-1-one.
This is a β-keto carboxylic acid. The ketone carbonyl is at C1. The carboxymethyl group is attached to C2. The carbon of the −CH2− group is α to the carboxylic acid carbon. The carbon C2 of the ring is attached to this −CH2− group, so C2 is β to the carboxylic acid carbon. C2 is also α to the ketone carbonyl. Thus, the ketone carbonyl is on the β-carbon relative to the carbon α to the carboxylic acid carbon. Therefore, this is a β-keto carboxylic acid.
Step 2: Heating (Δ).
β-Keto carboxylic acids undergo decarboxylation upon heating, losing CO2.
The decarboxylation of 2-(carboxymethyl)cyclopentan-1-one leads to the loss of the −COOH group and the formation of a methyl group at the position where the −CH2COOH group was attached, but with the loss of the carbon from the −COOH group. The carbon that was part of the −CH2− group becomes a methyl group.
So, the −CH2COOH group at C2 is replaced by a −CH3 group at C2.
The product after decarboxylation is 2-methylcyclopentan-1-one.
Step 3: Reduction with LiAlH4.
LiAlH4 is a strong reducing agent that reduces ketones to secondary alcohols.
Reduction of 2-methylcyclopentan-1-one with LiAlH4 reduces the ketone group at C1 to a secondary alcohol.
The product is 2-methylcyclopentan-1-ol.
The final product is 2-methylcyclopentan-1-ol.
Explanation of the solution: The starting nitrile undergoes acidic hydrolysis to form a β-keto carboxylic acid. The β-keto carboxylic acid then undergoes decarboxylation upon heating to form a ketone. Finally, the ketone is reduced to a secondary alcohol using LiAlH4.
Let's reconfirm the decarboxylation of β-keto carboxylic acids. The mechanism involves a cyclic transition state where the enol form is formed, followed by tautomerization to the ketone. This process removes the carboxyl group as CO2. In 2-(carboxymethyl)cyclopentan-1-one, the −CH2COOH group is attached to the α-carbon of the ketone. The carbon of the −CH2− group is α to the −COOH group. The carbon C2 of the ring is β to the −COOH group. The ketone is at C1, which is α to C2. So the ketone is on the β-carbon relative to the carbon α to the −COOH. This is indeed a β-keto carboxylic acid.
