Question
Question: The given reaction is the transformation of 1,2,2-tris(hydroxymethyl)cyclopropane to benzene under a...
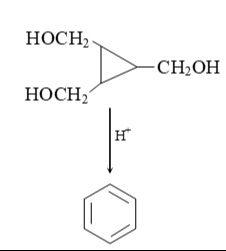
The given reaction is the transformation of 1,2,2-tris(hydroxymethyl)cyclopropane to benzene under acidic conditions. Explain the reaction.
Benzene
Solution
Under acidic conditions, the hydroxyl groups are protonated and eliminated as water, leading to the formation of carbocations. The cyclopropane ring opens, and the molecule undergoes a series of rearrangements to form the stable aromatic structure of benzene. The driving force for the reaction is the formation of the highly stable aromatic ring and the removal of water.
The overall reaction is a dehydration and rearrangement reaction. The six carbon atoms from the starting material are rearranged to form the six-membered ring of benzene. The 12 hydrogen atoms and 3 oxygen atoms in the starting material become 6 hydrogen atoms in benzene and 3 molecules of water (6 hydrogen atoms and 3 oxygen atoms).
A plausible mechanism involves a series of protonations, dehydrations, ring openings, and rearrangements that ultimately lead to the formation of a six-membered ring and removal of all oxygen atoms. The formation of benzene, an aromatic compound, is a highly favorable process due to its stability.
