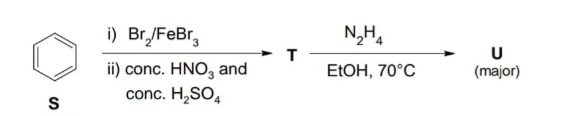
Question
Question: The reaction sequence starts with benzene (S). Step 1: Bromination of benzene. Benzene reacts with ...
The reaction sequence starts with benzene (S).
Step 1: Bromination of benzene. Benzene reacts with Br2 in the presence of FeBr3 (Lewis acid catalyst) to undergo electrophilic aromatic substitution, yielding bromobenzene.
S: Benzene Benzene + Br2/FeBr3 → Bromobenzene
Step 2: Nitration of bromobenzene. Bromobenzene reacts with a mixture of concentrated HNO3 and concentrated H2SO4 (nitrating mixture) to undergo electrophilic aromatic substitution (nitration). Bromine is an ortho, para-director. The major product of nitration of bromobenzene is para-bromonitrobenzene. Bromobenzene + conc. HNO3/conc. H2SO4 → para-bromonitrobenzene (major) + ortho-bromonitrobenzene T: para-bromonitrobenzene
Step 3: Reaction of para-bromonitrobenzene with hydrazine (N2H4) in ethanol at 70∘C. Para-bromonitrobenzene is an activated aryl halide due to the presence of the strongly electron-withdrawing nitro group at the para position to the bromine. This makes the carbon atom bearing the bromine susceptible to nucleophilic aromatic substitution (SNAr). Hydrazine is a nucleophile. The reaction is likely a nucleophilic aromatic substitution where the bromide leaving group is replaced by the hydrazino group (-NHNH2). T (para-bromonitrobenzene) + N2H4 → U (major)
The SNAr reaction proceeds via the addition-elimination mechanism. The nucleophile (hydrazine) attacks the carbon bearing the leaving group (bromine), forming a Meisenheimer complex. The electron-withdrawing nitro group stabilizes the negative charge in the intermediate. Then, the leaving group (bromide) is eliminated, restoring aromaticity. The nucleophile is N2H4. One of the nitrogen atoms attacks the carbon with bromine.
-
H2N-NH2 →
-
HBr
The major product U is 4-nitrophenylhydrazine.
While hydrazine can also act as a reducing agent and reduce nitro groups, the conditions (ethanol, 70∘C, without a catalyst) are more favorable for nucleophilic aromatic substitution of an activated aryl halide by a nucleophile like hydrazine. Reduction of the nitro group to an amino group by hydrazine typically requires different conditions or catalysts. Therefore, the major reaction is the nucleophilic substitution of bromine by hydrazine.
The final product U is 4-nitrophenylhydrazine.
4-nitrophenylhydrazine
Solution
Benzene undergoes bromination to form bromobenzene. Bromobenzene undergoes nitration, and the major product is para-bromonitrobenzene (T) due to the directing effect of bromine. Para-bromonitrobenzene is an activated aryl halide, and it undergoes nucleophilic aromatic substitution with hydrazine (N2H4) where the bromine is substituted by the hydrazino group (-NHNH2). The major product (U) is 4-nitrophenylhydrazine.
