Question
Question: The figure shows the reaction of 3-chlorocyclopentene with diethyl malonate in the presence of NaOEt...
The figure shows the reaction of 3-chlorocyclopentene with diethyl malonate in the presence of NaOEt to form X, and then X reacts with urea to form Y. Which of the following is a correct statement?
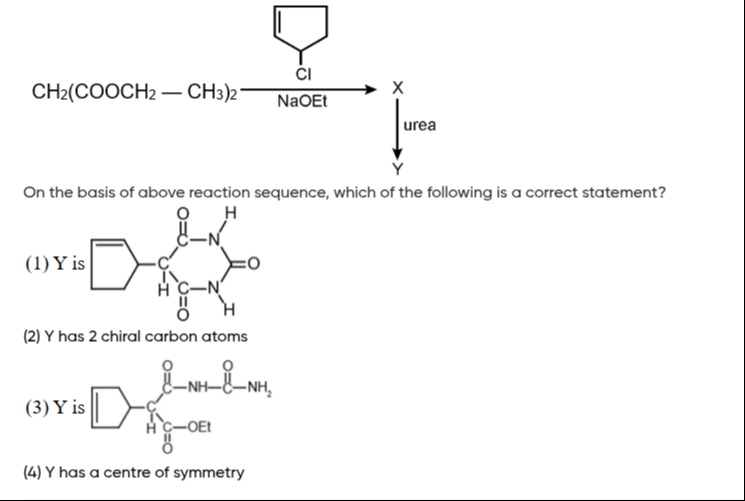
Y is [structure of barbituric acid derivative with cyclopent-3-en-1-yl group]
Y has 2 chiral carbon atoms
Y is [structure of an acyclic compound]
Y has a centre of symmetry
(1) and (2)
Solution
Step 1: Formation of X. 3-chlorocyclopentene reacts with diethyl malonate in the presence of NaOEt via nucleophilic substitution to form diethyl (cyclopent-3-en-1-yl)malonate (X).
Step 2: Formation of Y. X reacts with urea in a cyclocondensation reaction to form a barbituric acid derivative. The cyclopent-3-en-1-yl group is attached to the C5 position of the barbituric acid ring. This structure is correctly represented in option (1).
Chirality analysis:
- The C5 carbon of the barbituric acid ring is bonded to a hydrogen atom, the cyclopent-3-en-1-yl group, and two different parts of the cyclic urea structure, making it a chiral center.
- In the cyclopent-3-en-1-yl group, the carbon atom attached to the barbituric acid ring (let's call it C1 of the cyclopentene) is bonded to the barbituric acid moiety, a hydrogen atom, and two different paths around the ring (C1-C2-C3=C4-C5-C1 vs C1-C5-C4=C3-C2-C1). Due to the double bond at C3=C4, these paths are different, making C1 a chiral center.
Therefore, Y has 2 chiral carbon atoms, confirming option (2). Option (3) is incorrect as Y is a cyclic compound. Option (4) is incorrect as the molecule lacks symmetry due to the chiral centers and the unsymmetrical substituent.
