Question
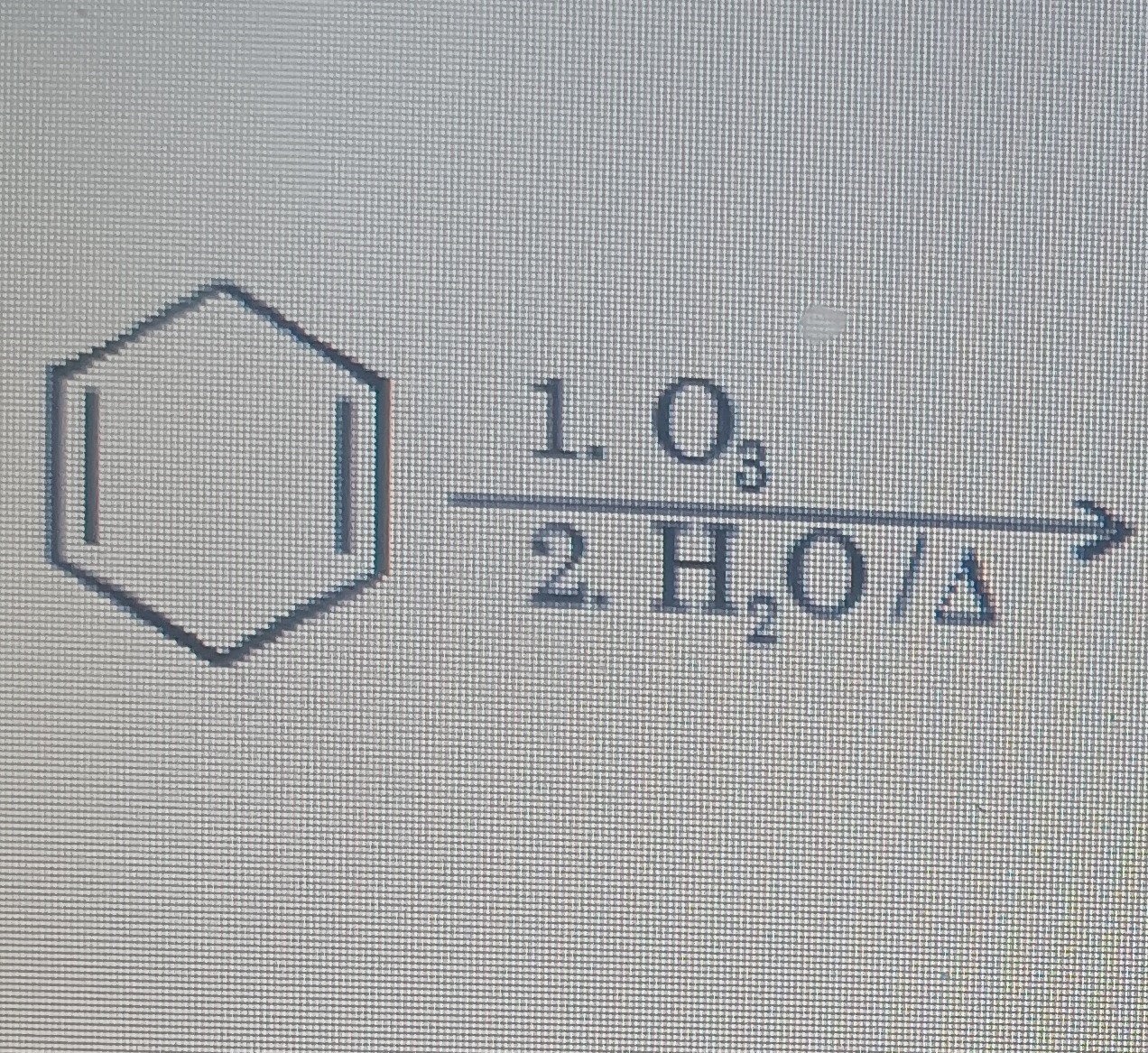
Question: The given reaction is the ozonolysis of 1,3-cyclohexadiene followed by oxidative workup ($H_2O/\Delt...
The given reaction is the ozonolysis of 1,3-cyclohexadiene followed by oxidative workup (H2O/Δ). Ozonolysis cleaves the carbon-carbon double bonds. The structure of 1,3-cyclohexadiene is a six-membered ring with double bonds at positions 1-2 and 3-4.
Hexanedial
Glyoxal and Succinaldehyde
Adipic acid
Cyclohexane-1,4-dione
Glyoxal and Succinaldehyde
Solution
Ozonolysis cleaves carbon-carbon double bonds. Oxidative workup converts the resulting fragments into carbonyl compounds. In 1,3-cyclohexadiene, the double bonds are at positions 1-2 and 3-4. Cleavage of the 1-2 double bond yields two carbonyl groups (one at carbon 1, one at carbon 2). Cleavage of the 3-4 double bond yields two carbonyl groups (one at carbon 3, one at carbon 4). The carbons 5 and 6 are connected by a single bond. This results in the formation of two separate molecules: glyoxal (ethanedial, OHC-CHO) from the C1-C2 cleavage and succinaldehyde (butanedial, OHC-CH2-CH2-CHO) from the C3-C4 cleavage and the connection between C5 and C6.
