Question
Question: The figure shows the starting material and two possible products. Identify the correct products form...
The figure shows the starting material and two possible products. Identify the correct products formed from the enolization of 2-methylcyclohexanone.
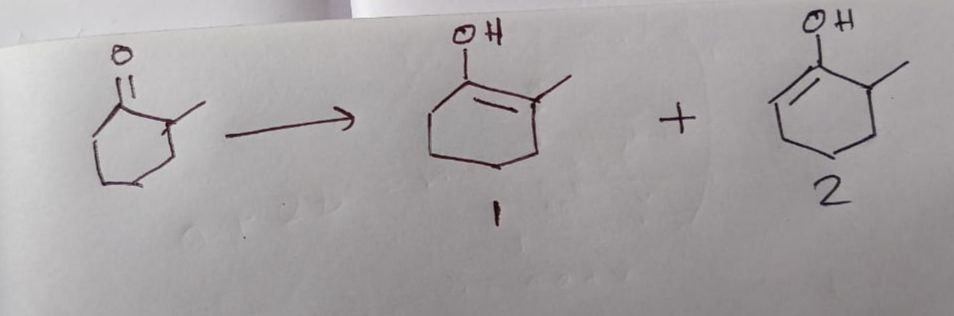
Product 1 only
Product 2 only
Both Product 1 and Product 2
Neither Product 1 nor Product 2
Product 2 only
Solution
Enolization of 2-methylcyclohexanone involves the removal of a proton from an alpha-carbon, leading to the formation of a double bond between the carbonyl carbon and the alpha-carbon, with a hydroxyl group on the carbonyl carbon.
2-methylcyclohexanone has two alpha-carbons:
- The carbon at position 2 (bearing the methyl group and one hydrogen).
- The carbon at position 6 (bearing two hydrogens).
Enolization at position 2: Removal of a proton from C2 forms a double bond between C1 and C2. The resulting enol is 2-methylcyclohex-1-en-1-ol. This corresponds to Product 2. This enol is thermodynamically favored due to the trisubstituted double bond and is also generally favored kinetically when enolization occurs at the more substituted alpha-carbon.
Enolization at position 6: Removal of a proton from C6 forms a double bond between C1 and C6. The resulting enol is 6-methylcyclohex-1-en-1-ol. However, the numbering convention for cyclohexenols typically places the double bond between C1 and C2. If the double bond is between C1 and C6, and the hydroxyl is on C1, the methyl group remains at C2. This would lead to 2-methylcyclohex-1-en-1-ol, which is the same as Product 2.
Product 1 is 3-methylcyclohex-1-en-1-ol. This product would be formed by enolization of 3-methylcyclohexanone, not 2-methylcyclohexanone. Therefore, Product 1 is not a direct enolization product of 2-methylcyclohexanone.
Thus, only Product 2 is formed from the enolization of 2-methylcyclohexanone.
