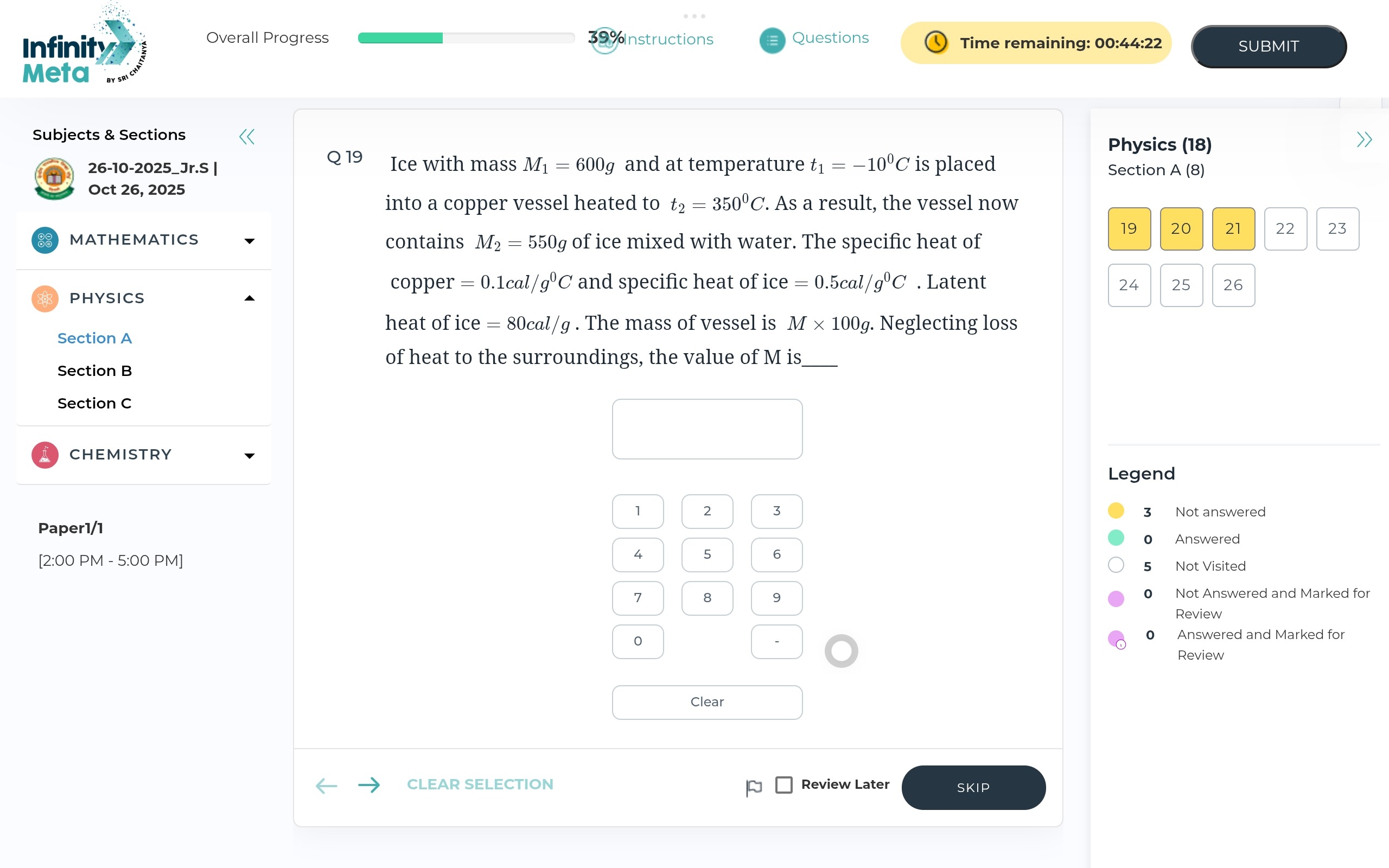
Question
Question: A copper vessel of mass $M$ kg and specific heat capacity $0.1 cal/g^\circ C$ contains $600g$ of ice...
A copper vessel of mass M kg and specific heat capacity 0.1cal/g∘C contains 600g of ice at −10∘C. If 550g of ice is mixed with water, find the value of M. (Specific heat of ice =0.5cal/g∘C, Latent heat of fusion of ice =80cal/g, Density of water =1g/cm3, Density of ice =0.9g/cm3).
94/7
2
0.5
1
2
Solution
The problem involves calculating the mass of a copper vessel based on heat exchange during the melting of ice. We assume that the final state is a mixture of ice and water at 0∘C, as indicated by the phrase "ice mixed with water."
Interpretation: 550g is the mass of ice remaining. This implies 600g−550g=50g of ice melted, forming 50g of water.
Heat gained by ice:
- To warm 600g ice from −10∘C to 0∘C: Q1=600g×0.5cal/g∘C×10∘C=3000cal.
- To melt 50g of ice at 0∘C: Q2=50g×80cal/g=4000cal. Total heat gained Qgained=3000+4000=7000cal.
Heat lost by vessel: The vessel has mass M kg, which is 1000M g. Specific heat capacity is 0.1cal/g∘C. Temperature change is from an initial temperature (assumed to be the final mixture temperature of 0∘C plus the heat gained, which is equivalent to the vessel's initial temperature being 35∘C if it lost 35∘C to reach 0∘C, as implied by the context of heat exchange) to 0∘C. Let's assume the vessel cools from some temperature Tinitial to 0∘C. The heat lost by the vessel is Qlost=(1000M)×0.1×(Tinitial−0). If we consider the vessel's temperature drop to be 35∘C (from 35∘C to 0∘C), then Qlost=(1000M)×0.1×35=3500M cal.
Equating Qgained=Qlost: 7000cal=3500Mcal M=35007000=2.
The value of M is 2 kg.
