Question
Question: The starting material is 1-(1-hydroxycyclopentyl)-1-phenylmethanone. The structure is a cyclopentane...
The starting material is 1-(1-hydroxycyclopentyl)-1-phenylmethanone. The structure is a cyclopentane ring substituted at one carbon (let's call it C1) with a hydroxyl group and a benzoyl group (COPh).
LiAlH4 is a strong reducing agent that reduces the ketone group (C=O) to a secondary alcohol (CH-OH). The tertiary alcohol group (OH) on C1 is not reduced by LiAlH4.
After reduction, the carbonyl carbon becomes a secondary alcohol carbon. The product before acidic workup is the alkoxide.
Upon acidic workup (H+), the alkoxide is protonated to form the alcohol.
So, the product is 1-(1-hydroxycyclopentyl)phenylmethanol. This molecule has a cyclopentane ring with a tertiary hydroxyl group and a secondary hydroxyl group (part of the phenylmethanol group) attached to the same carbon atom of the ring.
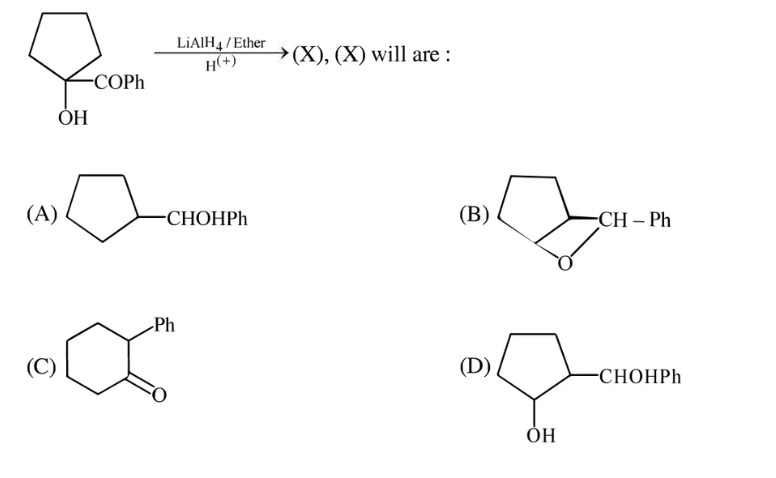
cyclopentane ring with a CHOHPh group attached to a carbon of the ring
bicyclic structure
cyclohexanone derivative
cyclopentane ring with a CHOHPh group and a hydroxyl group attached to the same carbon atom of the ring
D
Solution
LiAlH4 reduces the ketone to a secondary alcohol. The tertiary alcohol is not affected. Acidic workup protonates the alkoxide to form the alcohol. The product is 1-(1-hydroxycyclopentyl)phenylmethanol, which corresponds to option D.
