Question
Question: The reaction of an epoxide with lithium dimethylcuprate followed by hydrolysis yields: ...
The reaction of an epoxide with lithium dimethylcuprate followed by hydrolysis yields:
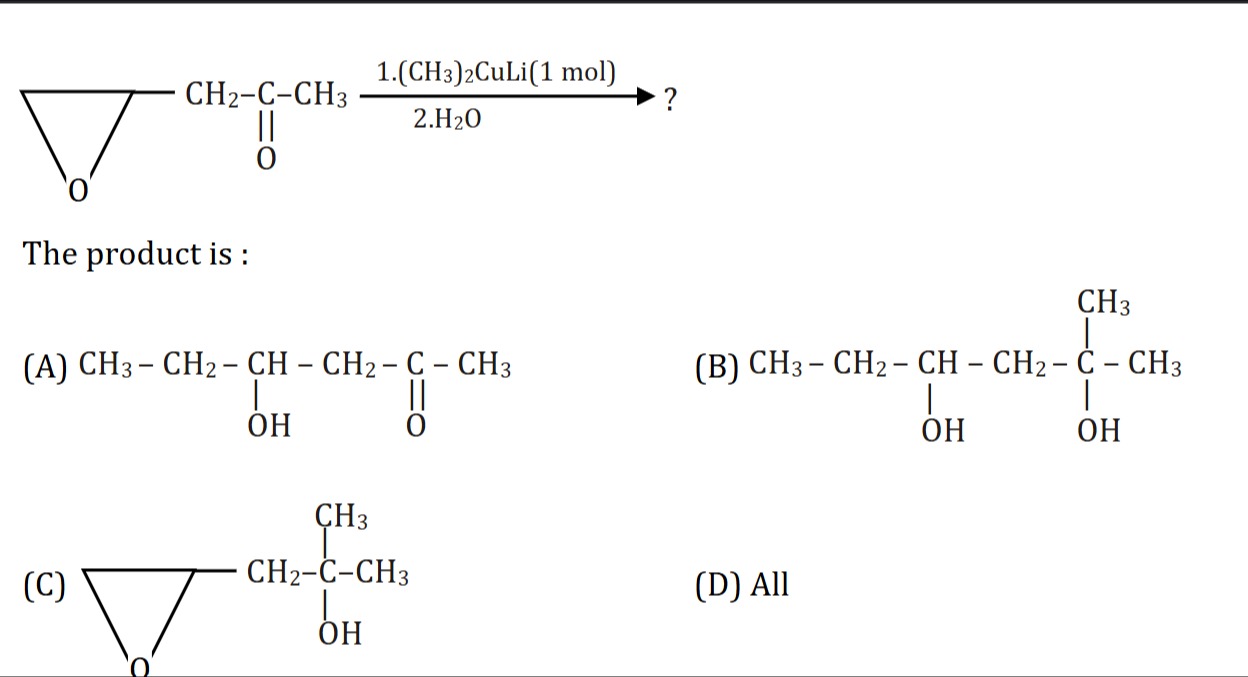
CH3 - CH2 - CH - CH2 - C - CH3 | || OH O
CH3 - CH - CH2 - CH2 - C - CH3 | || OH O
CH3 - CH2 - CH2 - CH - C - CH3 | || OH O
CH3 - CH2 - CH - CH2 - CH2 - C - CH3 | || OH O
CH3 - CH2 - CH - CH2 - C - CH3 | || OH O
Solution
Lithium dimethylcuprate ((CH3)2CuLi) is a Gilman reagent, a soft nucleophile that attacks the less substituted carbon of an epoxide. The epoxide in question is attached to a -CH2-C(=O)-CH3 group. The less substituted carbon of the epoxide is the terminal CH2. Thus, a methyl group attacks this carbon, opening the epoxide ring. Hydrolysis then protonates the alkoxide to form an alcohol. The resulting product is 4-hydroxyhexan-2-one.
