Question
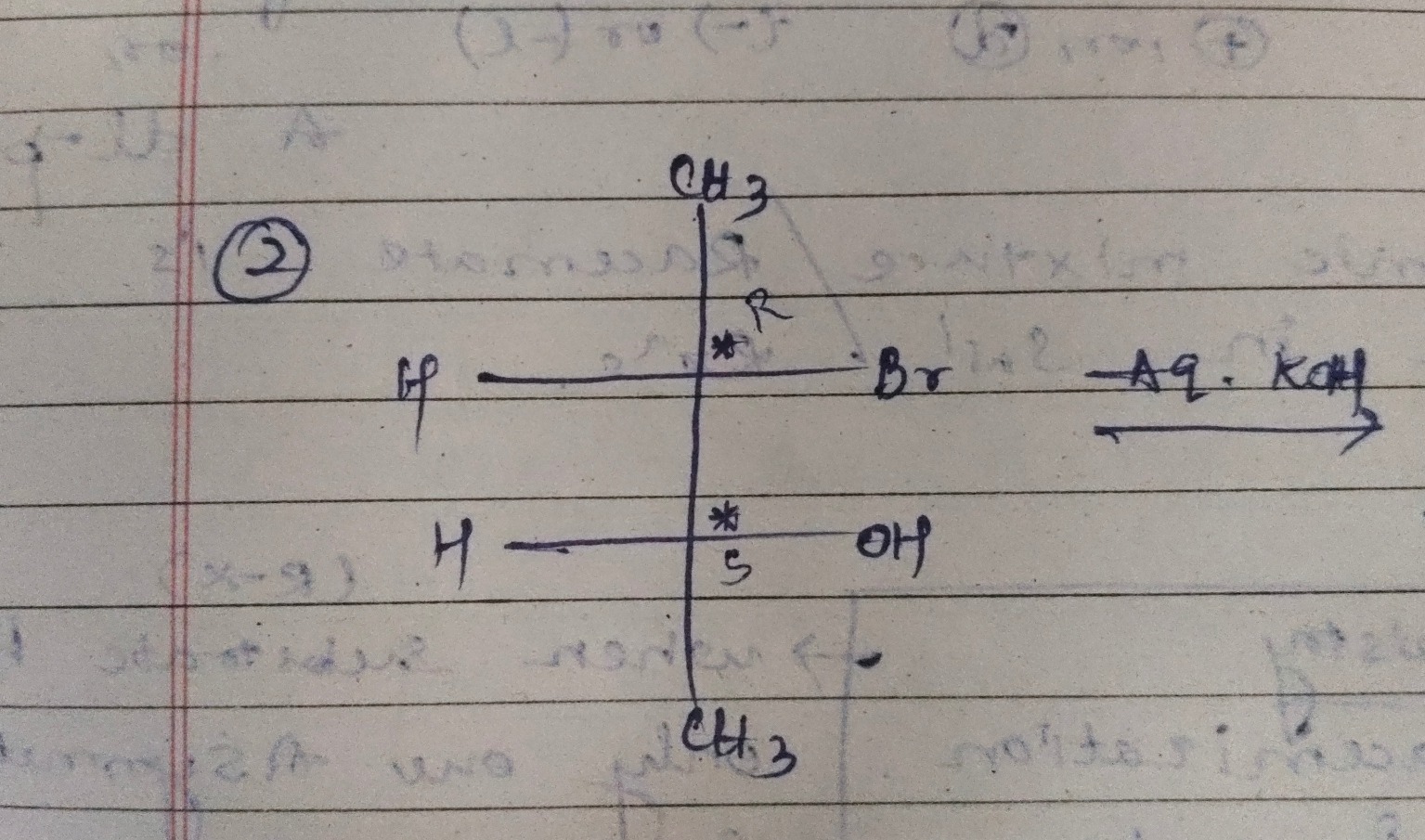
Question: The given reaction involves a substrate with two chiral centers, a bromine atom as a leaving group, ...
The given reaction involves a substrate with two chiral centers, a bromine atom as a leaving group, and aqueous KOH as the reagent. Aqueous KOH is a source of the nucleophile OH⁻ and a base. The reaction at the carbon bearing the bromine atom is expected to be a nucleophilic substitution. Given that it's a secondary halide in aqueous conditions with a strong nucleophile, an SN2 reaction is the most probable pathway.
The SN2 reaction proceeds with inversion of configuration at the carbon atom where the substitution occurs. The bromine atom is located at the top chiral center, which is labeled as R. Therefore, upon nucleophilic substitution by OH⁻, the configuration at this center will invert from R to S.
The bottom chiral center has a hydroxyl group. Under the basic conditions of the reaction, the hydroxyl group is not a good leaving group, so it is unlikely to be involved in substitution or elimination reactions. Thus, the configuration at the bottom chiral center remains unchanged. This center is labeled as S.
Combining these two points, the product will have an S configuration at the top chiral center and an S configuration at the bottom chiral center. The bromine atom will be replaced by a hydroxyl group. Therefore, the product is (2S, 3S)-butane-2,3-diol.
(2S, 3S)-butane-2,3-diol
(2R, 3S)-butane-2,3-diol
(2S, 3R)-butane-2,3-diol
(2R, 3R)-butane-2,3-diol
(2S, 3S)-butane-2,3-diol
Solution
The SN2 reaction proceeds with inversion of configuration. The reactant has a bromine at the top chiral center with R configuration and a hydroxyl group at the bottom chiral center with S configuration. In an SN2 reaction, the nucleophile (OH⁻) attacks the carbon bearing the leaving group (Br) from the backside, leading to an inversion of stereochemistry at that center. Thus, the R configuration at the top chiral center becomes S. The bottom chiral center with the hydroxyl group is not involved in the reaction, so its S configuration remains unchanged. Therefore, the product is (2S, 3S)-butane-2,3-diol.
