Question
Question: ...
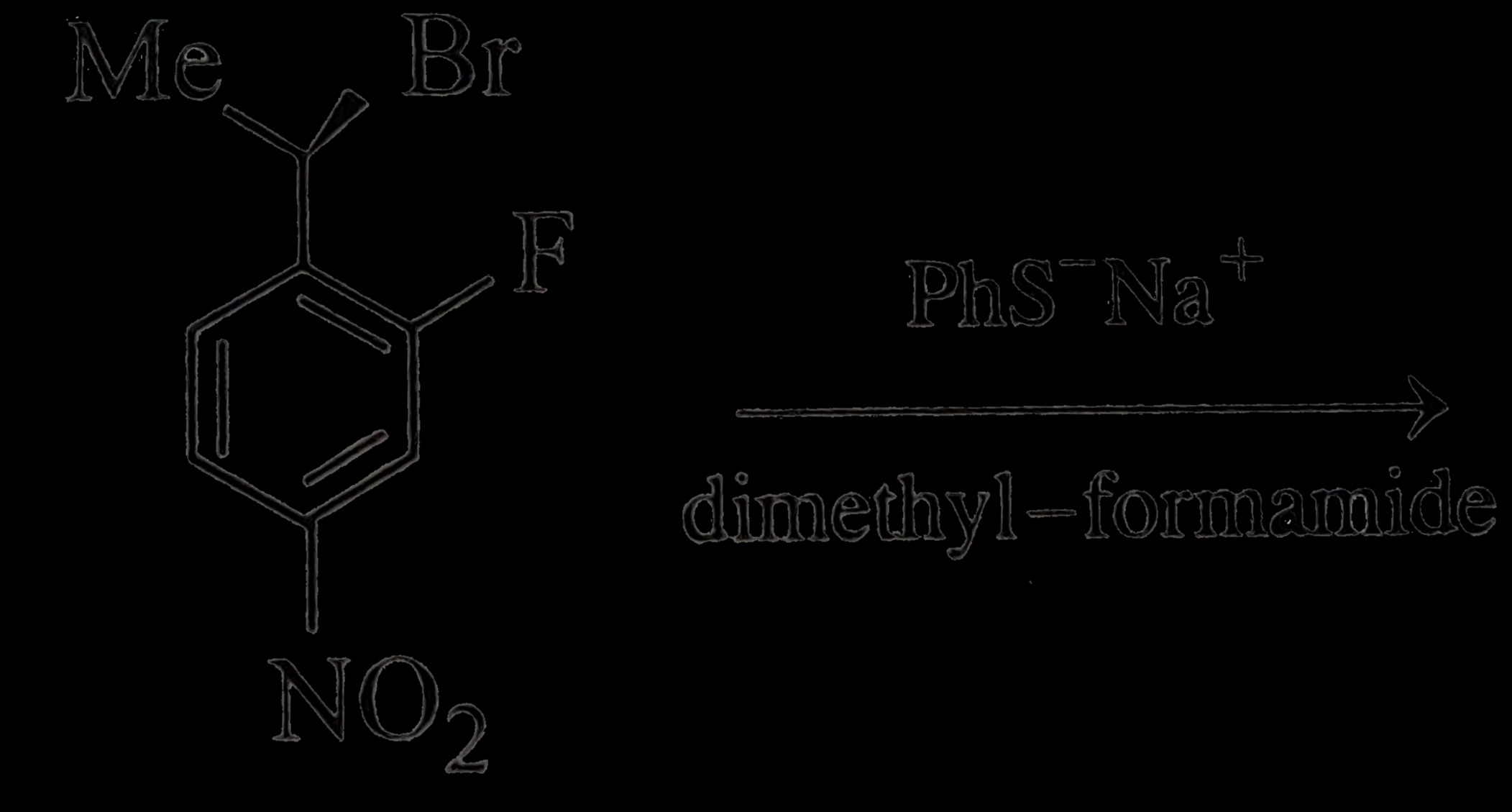
Answer
Product: 1-(1-(phenylthio)-1-methylethyl)-2-fluoro-4-nitrobenzene
Explanation
Solution
The reaction proceeds via nucleophilic substitution at the benzylic carbon. The strong nucleophile PhS− attacks the tertiary benzylic carbon, displacing the bromide ion. While a tertiary carbon usually disfavors S_N2 due to steric hindrance and a para-nitro group destabilizes S_N1 carbocation, the benzylic position is still a reactive site compared to the unactivated aryl fluoride.
