Question
Question: Few examples of the compounds formed by chemical bonding are given below. Mark the incorrect example...
Few examples of the compounds formed by chemical bonding are given below. Mark the incorrect example.
A. A molecule with central atom devoid of octet - BF3
B. A molecule with linear shape - CO2
C. A nonpolar covalent compound between two different atoms - CH4
D. A molecule which is V-shaped with a bond angle 104.5∘ - NH3
Solution
Draw the structures of the molecules using the Lewis dot structures. From the structures, determine the shapes of molecules BF3, CO2 and NH3.
A nonpolar covalent bond is formed when the atoms with no difference in their electronegativities form a bond by sharing their valence electrons.
Complete step by step answer:
Step 1:
Calculate the valence electrons of BF3.
The valence electrons of B are 3 and the valence electrons of F are 7. Thus,
Valence electrons of BF3 =(1×Valence electrons of B)+(3×Valence electrons of F)
=(1×3)+(3×7)
=3+21
Valence electrons of BF3 =24
The structure of BF3 is,
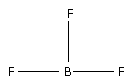
Three F atoms bond with one B atoms forming three bonds. As three bonds are formed, six electrons are involved in bonding. Thus, the remaining electrons are,
Remaining electrons =24−6=18
Place the remaining 18 electrons around the F atoms surrounding the central B atom. Thus,
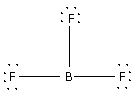
The 18 electrons get placed along the F atoms thus completing the octets of all the F atoms.
The B atom has only 6 electrons. Thus, it does not complete its octet.
Thus, the statement that a molecule with a central atom devoid of octet - BF3 is correct.
Step 2:
Calculate the valence electrons of CO2.
The valence electrons of C are 4 and the valence electrons of O are 6. Thus,
Valence electrons of CO2 =(1×Valence electrons of C)+(2×Valence electrons of O)
=(1×4)+(2×6)
=4+12
Valence electrons of CO2 =16
The structure of CO2 is,
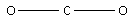
Remaining electrons =16−4=12
Place the remaining 12 electrons around the O atoms surrounding the central C atom. Thus,
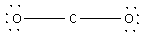
The 12 electrons get placed along the O atoms.
Thus, the structure of the CO2 molecule is linear.
Thus, a molecule with linear shape - CO2 is correct.
Step 3:
Covalent bonds are formed when the atoms share their valence electrons.
Carbon has four valence electrons and hydrogen has one valence electron.
Thus, carbon and hydrogen form covalent bonds by sharing their valence electrons.
The electronegativity difference between carbon and hydrogen is very low. Thus, the polarity of the bonds between carbon and hydrogen is very low.
Thus, the bonds between carbon and hydrogen are non-polar.
Thus, the statement a nonpolar covalent compound between two different atoms - CH4 is correct.
Step 4:
Calculate the valence electrons of NH3.
The valence electrons of N are 5 and the valence electrons of H are 1. Thus,
Valence electrons of NH3 =(1×Valence electrons of N)+(3×Valence electrons of H)
=(1×5)+(3×1)
=5+3
Valence electrons of NH3 =8
The structure of NH3 is,
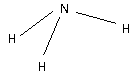
Three H atoms bond with one N atoms forming three bonds. As three bonds are formed, six electrons are involved in bonding. Thus, the remaining electrons are,
Remaining electrons =8−6=2
Place the remaining 2 electrons N atom. Thus,
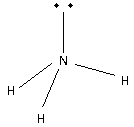
Thus, the shape of NH3 molecule is trigonal pyramidal.
The bonding pairs occupy less space than non-bonding electron pairs. Thus, the bond angle is 107∘.
Thus, the statement a molecule which is V-shaped with a bond angle 104.5∘ - NH3is incorrect.
Thus, the correct option is option (D).
Note: The electron lone pair occupies a space above the nitrogen atom. Thus, the shape of NH3 molecule is trigonal pyramidal. The bonding electron pairs occupy less space than non-bonding electron pairs and thus, the bond angle becomes 107∘.
