Question
Question: Ferric sulphate is represented by which formula? A. \(FeS{{O}_{4}}\) B. \(FeS{{O}_{3}}\) C. \[...
Ferric sulphate is represented by which formula?
A. FeSO4
B. FeSO3
C. Fe(SO4)2
D. Fe2(SO4)3
Solution
In order to mention the formula of ferric sulphate we are required to know the structure. Recall that the word ‘ferric’ indicates that the oxidation state of the Fe atom is +3.
Complete step by step answer:
First, we should look at the oxidation states of ions involved in the making of this molecule. The word ferric indicates that the oxidation state of the Fe ion is +3 and the word sulphate indicates that the formula of the ion present is SO4 we know that this ion has an oxidation state of -2 so we can rule out ‘option B’ since it has the SO3 ion. We know that the overall charge on the molecule is 0, so we can balance the charges to find the number of each ion involved in the molecule.
We see by trial and error method by considering all the options given that only in option ‘D. Fe2(SO4)3’ the overall charge comes out to be 0 (It is +1 in option A and -1 in option C).
The structure of Ferric sulphate is given below:
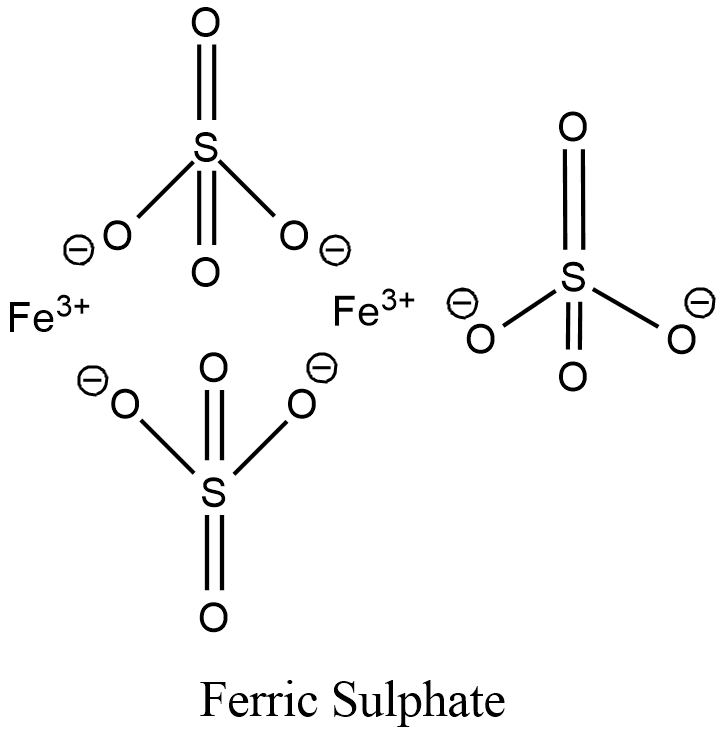
Hence, the answer is. Fe2(SO4)3’.
So, the correct answer is “Option B”.
Note: Remember that when the ion SO32− is involved, the word sulphite will be used in the naming of the molecule. The word that is indicative of the Fe2+ ion is ferrous. Keep this in mind while answering the questions.
