Question
Question: Find Alkane with least mol. wt (Comp. contain all 1°,2°,3°,4° Carbon) In this question when alkane h...
Find Alkane with least mol. wt (Comp. contain all 1°,2°,3°,4° Carbon) In this question when alkane had 4 times 1° Carbon than 4°C sir said C14H30 is impossible under condition and asked to attach a screenshot/picture of structure and I want to confirm it, so here it is
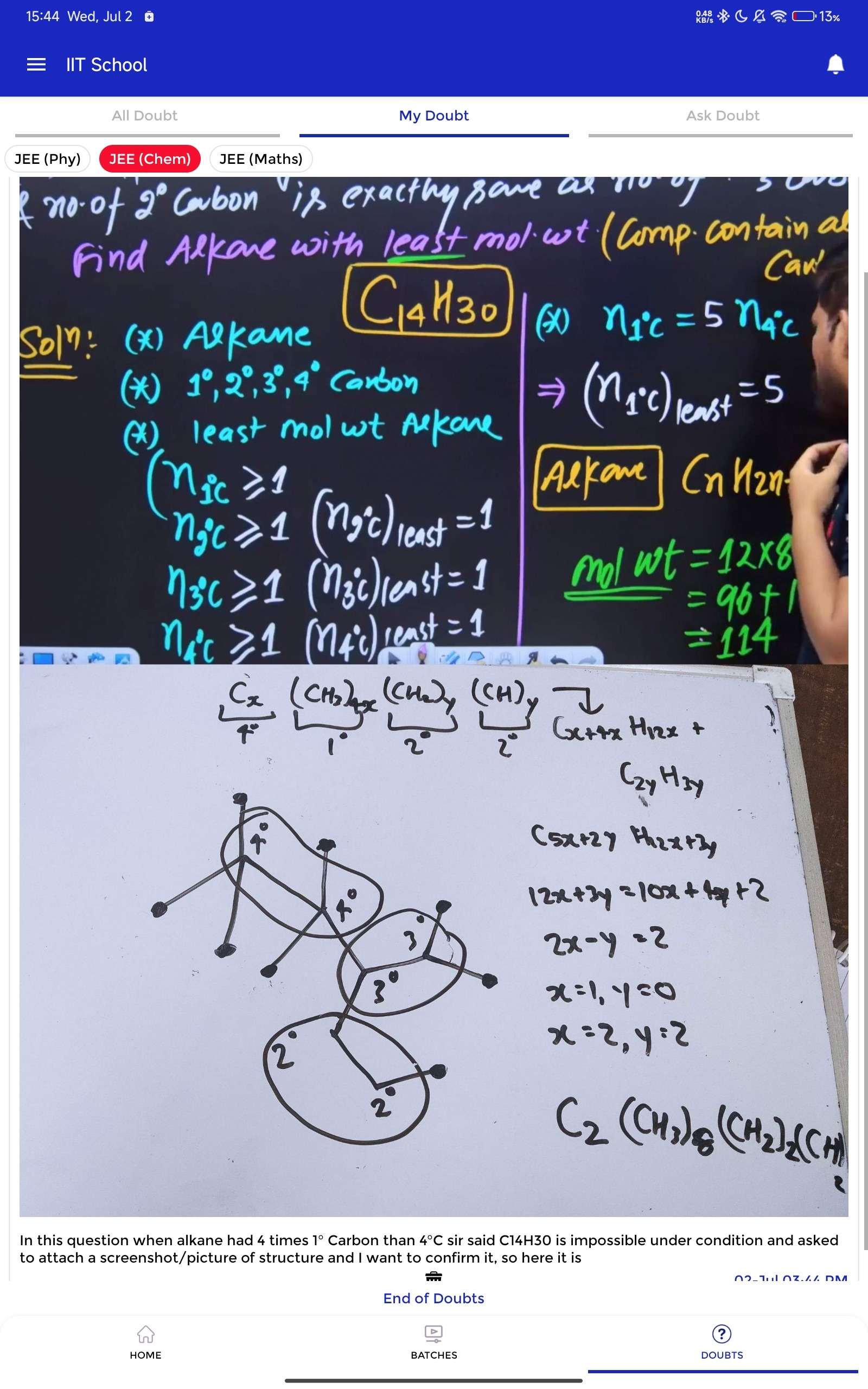
A C14H30 alkane is possible under the condition that n1∘C=4n4∘C and contains all types of carbons (1°, 2°, 3°, 4°). The number of carbons of each type would be n1=8,n2=2,n3=2,n4=2.
Solution
Let n1,n2,n3,n4 be the number of 1°, 2°, 3°, and 4° carbons, respectively. For an alkane CnH2n+2, the following relationships hold:
- Total carbons: n=n1+n2+n3+n4
- Relationship between carbon types: n1=n3+2n4+2 For C14H30, n=14. So, n1+n2+n3+n4=14.
The given condition is n1∘C=4n4∘C. Substitute this into the second relationship: 4n4=n3+2n4+2 n3=2n4−2
The problem states that the compound must contain all types of carbons, meaning n1≥1,n2≥1,n3≥1,n4≥1. From n3=2n4−2, for n3≥1, we must have 2n4−2≥1⟹2n4≥3⟹n4≥1.5. Since n4 must be an integer, the minimum value for n4 is 2.
Let's use n4=2: n3=2(2)−2=2. (This satisfies n3≥1) n1=4n4=4(2)=8. (This satisfies n1≥1)
Now substitute n1=8,n3=2,n4=2 into the total carbon equation: 8+n2+2+2=14 12+n2=14 n2=2. (This satisfies n2≥1)
So, for C14H30 under the given conditions, the number of each type of carbon must be: n1=8 n2=2 n3=2 n4=2
Let's verify the hydrogen count for these values: H=3n1+2n2+n3+0n4=3(8)+2(2)+2+0=24+4+2=30. This matches the hydrogen count for C14H30. Since a consistent set of non-zero integer values for n1,n2,n3,n4 is derived that satisfies all the given conditions and the general alkane formulas, such an alkane is theoretically possible. The existence of such a set of numbers guarantees the existence of at least one structural isomer.
The statement that C14H30 is impossible under the given conditions is incorrect.
