Question
Question: Explain Wurtz fittig reaction with example....
Explain Wurtz fittig reaction with example.
Solution
Organic reactions are the reactions involving organic compounds. Organic compounds are the compounds which contain carbons and its derivatives. Wurtz fittig reaction is also an example of an organic reaction.
Complete step by step answer:
Wurtz fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Aryl halides are also known as haloarene. Aryl halide is an aromatic compound in which one or more hydrogen atoms bonded to an aromatic ring are replaced by a halide. Alkyl halides are also known as haloalkanes. It is a group of chemical compounds derived from alkanes containing one or more halogens. Halogens are group 17 elements. An example of this reaction is as follows:
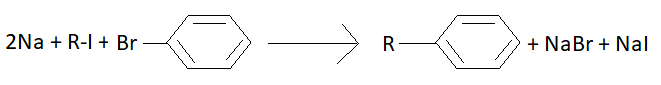
We can see that alkyl chain and an aromatic ring is joined. This is Wurtz fittig reaction.
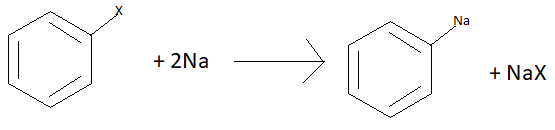
Mechanism of reaction: in the first step of this reaction halogen on the aromatic ring is replaced by sodium.
In the second step the alkyl ring gets attached to the aromatic ring and sodium forms a compound with a halogen atom.
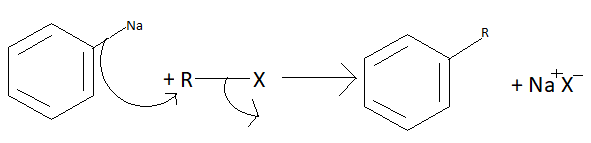
According to the radical mechanism:
In this type of mechanism, the halogen will take its electro and will form a compound with sodium and the aromatic ring will be left with its electron.
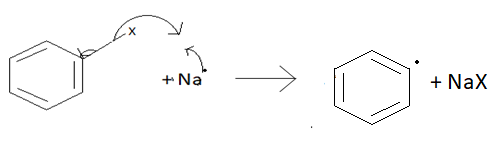
After that second sodium atom will form a compound with halogen of the alkyl chain. And alkyl chain will be left with its electron.
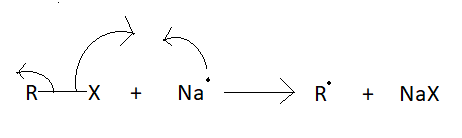
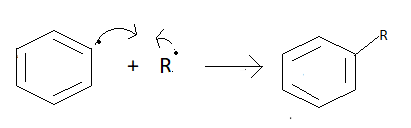
Then alkyl chain and aromatic ring will join with each other their one free electron.
Note:
In Wurtz fittig reaction aryl and alkyl compounds are joined together. Tischeko reaction is also a kind of organic reaction in which there is a disproportionation of aldehyde in presence of alkoxide. The alkoxide is the conjugate base of alcohol which consists of an organic compound bonded to negatively charged oxygen atom.
