Question
Question: Explain why the \[Be{H_2}\] molecule has a zero dipole moment although the \(Be - H\) Bonds are pola...
Explain why the BeH2 molecule has a zero dipole moment although the Be−H Bonds are polar.
Solution
Dipole moment is the product of magnitude of the partial charges developed on any covalently bonded atoms and the distance between the two atoms. Polar molecules are molecules in which distribution of charge between the covalently bonded atoms is not even due to the difference in the electro-negativity between the two bonded atoms.
Complete step by step answer:
We already know about the dipole moment. Polarity causes a molecule to have a dipole moment. There are some important points about dipole moment-
-In the case of polyatomic molecules the dipole moment not only depends upon the individual bonds but also on the spatial arrangement of various bonds in the molecule.
In such a case a molecule will have a dipole moment if the summation of all of the individual dipole moments is non-zero.
-Now, we know that in BeH2molecule beryllium is the central atom which has no lone pair of electrons, only two bond pairs in its valence shell. These pairs form a covalent bond with two hydrogen atoms and the structure we obtain is linear which is given as-H−Be−H .
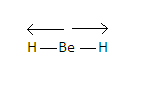
-Now we know that Be−H Bond is polar as partial positive charge develops on hydrogen atoms and partial negative charge develops on beryllium atoms because beryllium is more electronegative than hydrogen.Due to this the bonded atoms have a dipole moment which is directed towards the hydrogen atom.
-Similarly with the other hydrogen atom the central atom has a dipole moment in the direction of the hydrogen atom.
-Dipole moment of each Be−H bond is equal and opposite to each other. Therefore, they cancel out each other and the resultant dipole moment of the molecule becomes zero.
That is the reason why the BeH2 molecule has a zero dipole moment although the Be−H Bonds are polar.
Note:
Can also solve this question using the formula of the resultant dipole moment-
⇒ R=P2+Q2+2PQcosθ where P and Q are dipole moments of the polyatomic molecules.
Here the angle between the Be−Hbond is 180∘ and the dipole moments between the two Be−Hbonds are equal so we can write-
⇒ R=μ2+μ2+2μ×μ×cos180∘
We know thatcos180∘=−1 so on applying this we get-
⇒ R=μ2+μ2−2μ×μ
Now we know that (a−b)2=a2+b2+2ab so on applying this, we get-
⇒ R=(μ−μ)2
On solving, we get-
⇒ R=0
Hence the resultant dipole moment is zero.
